oxathiapiprolin
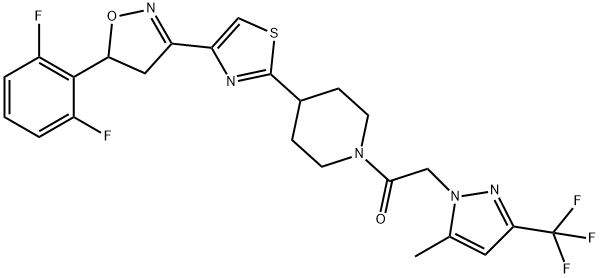
- CAS No.
- 1003318-67-9
- Chemical Name:
- oxathiapiprolin
- Synonyms
- oxathiapiprolin;Oxathioapiprolin;Oxathiapiprolin@100 μg/mL in Methanol;Ethanone, 1-[4-[4-[5-(2,6-difluorophenyl)-4,5-dihydro-3-isoxazolyl]-2-thiazolyl]-1-piperidinyl]-2-[5-methyl-3-(trifluoromethyl)-1H-pyrazol-1-yl]-
- CBNumber:
- CB63165610
- Molecular Formula:
- C24H22F5N5O2S
- Molecular Weight:
- 539.52
- MOL File:
- 1003318-67-9.mol
- Modify Date:
- 2023/5/15 10:43:34
| Boiling point | 613.9±65.0 °C(Predicted) |
|---|---|
| Density | 1.54±0.1 g/cm3(Predicted) |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 0.78±0.10(Predicted) |
| color | Off-White to Pale Yellow |
| EPA Substance Registry System | Ethanone, 1-[4-[4-[5-(2,6-difluorophenyl)-4,5-dihydro-3-isoxazolyl]-2-thiazolyl]-1-piperidinyl]-2-[5-methyl-3-(trifluoromethyl)-1H-pyrazol-1-yl]- (1003318-67-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H410 |
| Precautionary statements | P273-P391-P501 |
| Toxicity | Acute toxicity studies No acute toxicity observed Acute oral rat LD50 > 5000 mg kg−1 Acute dermal rat LD50 > 5000 mg kg−1 Acute inhalation rat LC50 > 5.1 mg kg−1 Rabbit eye or skin Not an irritant Dermal sensitization in guinea pigs Nonsensitizing |
oxathiapiprolin Chemical Properties,Uses,Production
Description
Oxathiapiprolin is now registered in numerous countries around the world, including Australia, Canada, China, Japan, and the United States, with the reviews confirming a highly favorable toxicological and environmental profile.
Physical properties
Melting point 146–148 °C (Form B)
Water solubility 0.175 mg l−1
log Kow 3.67
Kfoc 8368 g kg−1
Vapor pressure (20 °C) 1.14 × 10−6 Pa
Hydrolysis Stable
Aqueous photolysis DT50 ~ 15 d
Degradation in soil DT50 ~ 90 d
Pharmacology
Oxathiapiprolin is the first member of a new class of highly active oomycete fungicides, the piperidinyl thiazole isoxazolines. It is effective at extremely low use rates and shows excellent preventative and curative efficacy, excellent antisporulant properties, and long residual control with protection of new growth. It was discovered using a high-throughput screening approach with diverse structural classes and optimized to this high potency chemotype by conformationally restricting the benzyl amide moiety using an isoxazoline ring as an amide bioisostere. Oxathiapiprolin has a novel site of action, binding strongly to an OSBP with an, as yet, unknown cellular function. Oxathiapiprolin has the perfect combination of attributes to provide outstanding oomycete disease control and is being developed globally as DuPont? Zorvec? disease control. We expect that products containing oxathiapiprolin will become valuable tools for growers in their efforts to combat these diseases and sustainably and responsibly meet the growing global food production needs.
oxathiapiprolin Preparation Products And Raw materials
Raw materials
Preparation Products
Related articles
- How is Oxathiapiprolin synthesised?
- Oxathiapiprolin is synthesised using 4- cyanopiperidine as a raw material by chemical reaction.
- Feb 4,2024





