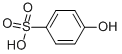
PHENOLSULFONIC ACID
- CAS No.
- 1333-39-7
- Chemical Name:
- PHENOLSULFONIC ACID
- Synonyms
- phenolsulfonic;SULFOCARBOLIC ACID;PHENOLSULFONIC ACID;phenolsulfonicacidliquid;Phenolsulfonic acid (65%);hydroxybenzenesulfonicacid;hydroxy-benzenesulfonicaci;hydroxy-Benzenesulfonicacid;hydroxybenzenesulphonic acid;Benzenesulfonic acid, hydroxy-
- CBNumber:
- CB6332613
- Molecular Formula:
- C6H6O4S
- Molecular Weight:
- 174.17
- MOL File:
- 1333-39-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/4 17:34:33
| Boiling point | 275.14°C (rough estimate) |
|---|---|
| Density | 1.384 (estimate) |
| refractive index | 1.5500 (estimate) |
| storage temp. | Store below +30°C. |
| EPA Substance Registry System | Phenolsulfonic acid (1333-39-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS05 |
|---|---|
| Signal word | Danger |
| Hazard statements | H314-H302-H312 |
| Precautionary statements | P280-P302+P352-P312-P322-P363-P501-P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501-P264-P270-P301+P312-P330-P501 |
| Hazard Codes | C |
| Risk Statements | 22-34 |
| Safety Statements | 26-36/37/39-45 |
PHENOLSULFONIC ACID Chemical Properties,Uses,Production
Description
Phorate is a stable, clear, pale yellow mobile liquid. Phorate is soluble and miscible with carbon
tetrachloride, dioxane, vegetable oils, xylene, alcohols, ethers, and esters. Phorate get hydrolysed
in the presence of moisture and by alkalis, toxic oxides of carbon, sulphur, and phosphorus.
Phorate may be used in foliar or soil treatments on alfalfa, barley, beans, brassicas, coffee,
corn, cotton, grapes, hops, lettuce, oats, peanuts, potatoes, rice, sorghum, soybeans, sugarcane,
sugar beets, tomatoes, watermelon, wheat, and on ornamentals and pine nursery stock. Phorate
has applications for the control of a wide variety of crop pests such as mites, aphids, greenbugs,
thrips, leafhoppers, sorghum shoot fly, leaf miners, corn rootworms, psyllids, cutworms,
Hessian fly, foliar nematodes, wireworms, flea beetles, whiteflies, pine tip moth, and others.
General Description
A yellowish liquid that becomes brown on exposure to air. Soluble in alcohol. Irritating to mucous membranes, skin, and eyes. Moderately toxic by ingestion. Used as a laboratory reagent, in water analysis and in the manufacture of pharmaceuticals. A mixture of ortho and para isomers.
Air & Water Reactions
Hygroscopic. Water soluble.
Reactivity Profile
PHENOLSULFONIC ACID is a strong acid. Reacts exothermically with chemical bases (for example: amines and inorganic hydroxides) to form salts. Dissolution in water or the dilution of a concentrated solutions with water may generate significant amounts of heat. Can serve as an oxidizing agent, but has a much weaker oxidizing action than sulfuric acid [Noller]. Can react with active metals, including iron and aluminum, and also many less active metals, to dissolve the metal and liberate hydrogen and/or toxic gases. Can initiate polymerization in certain classes of organic compounds. Reactions with cyanide salts and compounds release gaseous hydrogen cyanide. Flammable and/or toxic gases may be generated by reactions with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and strong reducing agents. Additional gas-generating reactions occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and even carbonates: the carbon dioxide gas from the last is nontoxic but the heat and spattering from the reaction can be troublesome. Can catalyze (increase the rate) of chemical reactions.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
PHENOLSULFONIC ACID Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Nico Orgo Marketing Pvt Ltd | +91-2249743857 +91-9004863178 | Maharashtra, India | 11 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| LEAP CHEM CO., LTD. | +86-852-30606658 | China | 24738 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 52927 | 58 | Inquiry |
| Combi-Blocks Inc. | 858 635 8950 | United States | 6632 | 69 | Inquiry |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 17691182729 18161915376 | China | 10011 | 58 | Inquiry |
| Guangdong wengjiang Chemical Reagent Co., Ltd. | 0751-2815987 13927875076 | China | 9977 | 58 | Inquiry |
| Hangzhou Yaoruiyicheng Biological Medicine Co., Ltd | 18072846723 | China | 18924 | 58 | Inquiry |
| QUALITY CONTROL SOLUTIONS LTD. | 13670046396 | China | 18865 | 58 | Inquiry |





