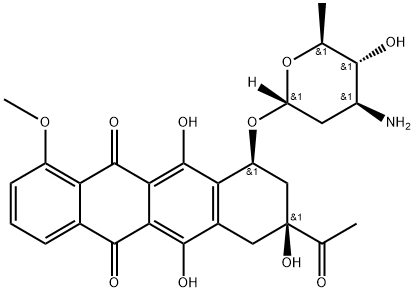
Epirubicin EP Impurity F
- CAS No.
- 57918-24-8
- Chemical Name:
- Epirubicin EP Impurity F
- Synonyms
- 4’-epi-Daunorubicin;Epirubicin EP Impurity F;Epirubicin hydrochloride EP Impurity F(epi-Daunorubicin);5,12-Naphthacenedione, 8-acetyl-10-[(3-amino-2,3,6-trideoxy-α-L-arabino-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-, (8S,10S)-
- CBNumber:
- CB65479522
- Molecular Formula:
- C27H29NO10
- Molecular Weight:
- 527.53
- MOL File:
- 57918-24-8.mol
- Modify Date:
- 2023/9/6 16:57:38
| Boiling point | 770.0±60.0 °C(Predicted) |
|---|---|
| Density | 1.55±0.1 g/cm3(Predicted) |
| storage temp. | Amber Vial, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated) |
| form | Solid |
| pka | 7.39±0.60(Predicted) |
| color | Dark Red to Black |
| Stability | Light Sensitive |
| InChIKey | STQGQHZAVUOBTE-WXNQWCPFNA-N |
| SMILES | OC1=C2C(C3=CC=CC(OC)=C3C(=O)C2=C(O)C2[C@H](C[C@](O)(C(=O)C)CC1=2)O[C@]1([H])O[C@@H](C)[C@H](O)[C@@H](N)C1)=O |&1:18,20,28,31,33,35,r| |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H340-H312-H360-H332-H350-H302 |
| Precautionary statements | P280-P302+P352-P312-P322-P363-P501-P261-P271-P304+P340-P312-P264-P270-P301+P312-P330-P501 |
Epirubicin EP Impurity F Chemical Properties,Uses,Production
Uses
Daunorubicin (D194500) impurity. Daunomycin analog antitumor.
Uses
Epirubicin EP Impurity F is an impurity of the drug Epirubicin, which is a pharmaceutical impurity standard in accordance with the European Pharmacopoeia, which can be used as a reference to analyze the quality of the drug. It is often used in the pharmaceutical industry. Epirubicin is an anthracycline topoisomerase II inhibitor used as an adjuvant to treating axillary node metastases in patients who have undergone surgical resection of primary breast cancer.
Preparation
9.0 g of the raw material was suspended in 460 mL of purified water. Then the temperature was cooled to 0 ~ 5 °C, and 60 mL of 3.5% sodium hydroxide solution was added dropwise for 50 minutes. After the reaction was complete, the pH was adjusted to 5.0 with hydrochloric acid. The reaction solution was subsequently washed twice with 200 mL of dichloromethane and the aqueous phase was collected. 1200 mL of dichloromethane and 80 mL of methanol were added to the aqueous phase, and the pH was adjusted to 8.2 with a 0.1 mol/L sodium hydroxide solution. Collect the organic phase. The organic phase was concentrated to dryness under reduced pressure Daunorubicin7.48g.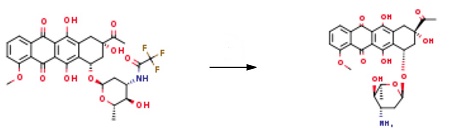
Epirubicin EP Impurity F Preparation Products And Raw materials
Epirubicin EP Impurity F Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| BOC Sciences | 16314854226; +16314854226 | United States | 19743 | 58 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
| China National Standard Pharmaceutical Corporation Limited | +8615391658522 | China | 11927 | 58 | Inquiry |
| Zhejiang Huida Biotech Co., LTD | 0571-89903882 13626641628 | China | 3656 | 58 | Inquiry |
| QUALITY CONTROL SOLUTIONS LTD. | 13670046396 | China | 18865 | 58 | Inquiry |
| Zhejiang Huida Biotech Co., LTD | 0571-0571-89903882 15990081639 | China | 3705 | 58 | Inquiry |
| Shanghai Kewel Chemical Co., Ltd. | 021-64609169 18901607656 | China | 9911 | 50 | Inquiry |
| Hubei Qingbei Yunyan Pharmaceutical Technology Co., Ltd | 18162595016 | China | 9887 | 58 | Inquiry |





