Imatinib mesylate
- CAS No.
- 220127-57-1
- Chemical Name:
- Imatinib mesylate
- Synonyms
- GLEEVEC;GLIVEC;Imatinib (Glivec);Gleevac;STI-571;CGP54148B;CGP 57148;CGP-57148B;Genfatinib;tinib Mesylate
- CBNumber:
- CB7173646
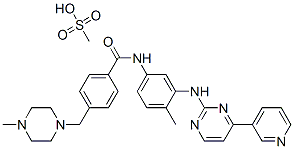
- Molecular Formula:
- C30H35N7O4S
- Molecular Weight:
- 589.71
- MOL File:
- 220127-57-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/21 22:41:43
| Melting point | 214-224°C |
|---|---|
| storage temp. | 2-8°C |
| solubility | H2O: soluble10mg/mL, clear |
| form | White solid |
| color | white to beige |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or water may be stored at -20°C for up to 3 months. |
| InChIKey | YLMAHDNUQAMNNX-UHFFFAOYSA-N |
| SMILES | C(NC1=CC=C(C)C(NC2=NC=CC(C3=CC=CN=C3)=N2)=C1)(=O)C1=CC=C(CN2CCN(C)CC2)C=C1.CS(O)(=O)=O |
| CAS DataBase Reference | 220127-57-1(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H361d | |||||||||
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | CV5520550 | |||||||||
| HS Code | 29350090 | |||||||||
| NFPA 704 |
|
Imatinib mesylate price More Price(6)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | SML1027 | Imatinib mesylate ≥98% (HPLC) | 220127-57-1 | 10MG | ₹4306.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | SML1027 | Imatinib mesylate ≥98% (HPLC) | 220127-57-1 | 100MG | ₹14652 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | CDS022105 | Imatinib mesylate | 220127-57-1 | 100MG | ₹7393.48 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 5.04595 | Bcr-abl Inhibitor IV, Imatinib - CAS 220127-57-1 - Calbiochem | 220127-57-1 | 50MG | ₹15740 | 2022-06-14 | Buy |
| TCI Chemicals (India) | I0936 | Imatinib Mesylate | 220127-57-1 | 100MG | ₹7900 | 2022-05-26 | Buy |
Imatinib mesylate Chemical Properties,Uses,Production
Description
In May 2001, the FDA approved imatinib as a new cancer drug after a record review time of just 2.5 months. Imatinib was launched as Gleevec in the US for chronic myelogenous leukemia (CML) in blast crisis, accelerated phase or chronic phase after interferon-alpha failure. This compound can be prepared by a four step sequence from a condensation of the 1-(3-pyridyl)ethanone with dimethyl formamide dimethylacetal, followed by successive cyclization with the methyl-nitrophenyl guanidine, hydrogenolysis and condensation with the benzoyl chloride of the methylpiperazine. Imatinib is the first of a new class of anticancer drugs that are specifically designed to target the molecular pathways involved (oncogenic event) in the development of disease. The Brc-Abl oncoprotein is a constitutively active tyrosine kinase that causes CML. Imatinib is a competitive inhibitor of this tyrosine kinase as well as Abl, Kit and the PDGFR kinases It binds to the ATP-binding site of the target kinase and prevents the transfer of phosphate from ATP to the tyrosine residues of various substrates and consequently blocks the proliferation of the leukemic cells. Phase II studies demonstrated that in chronic phase CML, over 90% of the patients had their leukocyte counts return to normal and 56% had a major cytogenic response. No phase III data is currently available. It is clear from the evidence available that imatinib has advantages over IFN-alpha, such as reduced toxicity, more rapid hematological response, higher rate of cytogenic response and oral administration. The drug is well tolerated, producing few side effects, classified as grade 1 nausea, muscle cramps, diarrhea, edema and vomiting. Imatinib is metabolized primarily by the CYP3A4 enzyme system and drugs capable of modulating this system would be expected to modify the patient's exposure. Novartis expects to launch imatinib for the treatment of gastrointestinal stromal tumors in 2002.
Chemical Properties
Off-White Solid
Uses
Imatinib Mesylate is orally bioavailability mesylate salt of Imatinib, which is a multi-target inhibitor of v-Abl, c-Kit and PDGFR with IC50 of 0.6 μM, 0.1 μM and 0.1 μM, respectively. Imatinib also known as Gleevec, Glivec, CGP-57148B, STI-571 & Imatinib
Definition
ChEBI: A methanesulfonate (mesylate) salt that is the monomesylate salt of imatinib. Used for treatment of chronic myelogenous leukemia and gastrointestinal stromal tumours.
General Description
Imatinib is available in 100- and 400-mg capsules for oraladministration and is indicated for the treatment of CML,gastrointestinal stromal tumors (GIST) that express Kit andacute lymphoplastic leukemia that is positive for thePhiladelphia chromosome.
Bioavailability of the agent is nearly 100% by the oralroute. The agent is highly protein bound and metabolized tothe N-desmethyl derivative by CYP3A4-mediated removalof the piperazinyl methyl group. The resulting metabolite issimilar to the parent in activity. Elimination occurs primarilyin the feces, and the terminal half-life is 18 hours forthe parent and 40 hours of the N-desmethyl metabolite.Resistant forms of the TK are known, which have alteredamino acids that prevent binding. In addition, there may beincreased levels of the kinase itself. The drug is also a substratefor Pgp and an additional efflux transporter known asbreast cancer resistance protein (BCRP), both of which removethe drug from the cell. These transporters are also inhibitedby the agent as well. Severe side effects include ascites,neutropenia, thrombocytopenia, skin rash, andpulmonary edema. Less serious side effects include nausea/vomiting, heartburn, and headache but overall, the agentis better tolerated than most other medications used in treatingthe disease.
Imatinib mesylate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| TAGOOR LABORATORIES PVT LTD | +919700187008 | AndhraPradesh, India | 93 | 58 | Inquiry |
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| AARTIA KEM SCIENCE | +91-8291072530 +91-8291072530 | Maharashtra, India | 70 | 58 | Inquiry |
| SNECOFRi Pvt Ltd | +91-9032850129 +91-9032850129 | Telangana, India | 404 | 58 | Inquiry |
| Gonane Pharma | +91-9819380043 +91-9819380043 | NaviMumbai, India | 192 | 58 | Inquiry |
| Cipla Ltd | +912224826000 | Maharashtra, India | 133 | 58 | Inquiry |
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| Vannsh Life Sciences Pvt Ltd | +91-4023555246 +91-9642555246 | Hyderabad, India | 15 | 58 | Inquiry |
| Chorus Labs Limited | +91-9640678999 +91-9640678999 | Hyderabad, India | 11 | 58 | Inquiry |
| HRV Global Life Sciences | +91-9820219686 +91-9820219686 | Telangana, India | 379 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| TAGOOR LABORATORIES PVT LTD | 58 |
| Jigs Chemical ltd | 58 |
| AARTIA KEM SCIENCE | 58 |
| SNECOFRi Pvt Ltd | 58 |
| Gonane Pharma | 58 |
| Cipla Ltd | 58 |
| Hetero Drugs Limited | 58 |
| Vannsh Life Sciences Pvt Ltd | 58 |
| Chorus Labs Limited | 58 |
| HRV Global Life Sciences | 58 |
Related articles
- Imatinib mesylate: Indications, Mechanism of Action and Side Effects
- Imatinib mesylate is an oral tyrosine kinase inhibitor used to treat chronic granulocytic leukaemia and certain types of cance....
- Sep 6,2024
- Imatinib mesylate: Tyrosine kinase inhibitor
- Gefitinib, an aniline quinazoline derivative, is an oral epidermal growth factor receptor’s (EGFR) tyrosine kinase inhibitor d....
- Oct 14,2019
220127-57-1(Imatinib mesylate)Related Search:
1of4
chevron_right




