Cobalt chloride
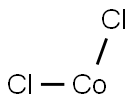
- CAS No.
- 7646-79-9
- Chemical Name:
- Cobalt chloride
- Synonyms
- CoCl2;COBALT(II) CHLORIDE;COBALT DICHLORIDE;COBALTOUS CHLORIDE;Cobalt(Ⅱ)chloride;Anhydrous cobalt chloride;COBALT(II) CHLORIDE, ANHYDROUS, BEADS, - 10 MESH, 99.9%;CobaL;t(II) chL;Cobatope-60
- CBNumber:
- CB8226062
- Molecular Formula:
- Cl2Co
- Molecular Weight:
- 129.84
- MOL File:
- 7646-79-9.mol
- Modify Date:
- 2024/8/28 13:53:27
| Melting point | 724 °C(lit.) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boiling point | 1049 °C | ||||||||||||||
| Density | 3.35 | ||||||||||||||
| vapor pressure | 40 mm Hg ( 0 °C) | ||||||||||||||
| Flash point | 500°C | ||||||||||||||
| storage temp. | Store below +30°C. | ||||||||||||||
| solubility | 585.9g/l soluble | ||||||||||||||
| form | beads | ||||||||||||||
| Specific Gravity | 3.356 | ||||||||||||||
| color | Pale blue | ||||||||||||||
| PH | pH (50g/l, 25℃) : >=3.0 | ||||||||||||||
| Water Solubility | soluble | ||||||||||||||
| Sensitive | Hygroscopic | ||||||||||||||
| Crystal Structure | CdCl2 type | ||||||||||||||
| crystal system | Three sides | ||||||||||||||
| Sublimation | 500 ºC | ||||||||||||||
| Merck | 14,2437 | ||||||||||||||
| Space group | R3m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits | ACGIH: TWA 0.02 mg/m3 | ||||||||||||||
| Stability | hygroscopic | ||||||||||||||
| CAS DataBase Reference | 7646-79-9(CAS DataBase Reference) | ||||||||||||||
| NIST Chemistry Reference | Cobalt dichloride(7646-79-9) | ||||||||||||||
| EPA Substance Registry System | Cobalt chloride (CoCl2) (7646-79-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H317-H334-H341-H350i-H360F-H410 | |||||||||
| Precautionary statements | P202-P273-P280-P301+P312-P302+P352-P308+P313 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 49-42/43-52/53-50/53-22-68-41-60-51/53 | |||||||||
| Safety Statements | 53-23-36/37-45-60-61-22-39-26 | |||||||||
| RIDADR | UN 2923 8/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | GF9800000 | |||||||||
| F | 9-21 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28273930 | |||||||||
| Toxicity | LD50 in mice, rats (mg/kg): 360.0, 171.0 orally; 92.6, 36.9 i.p.; 23.3, 4.3 i.v. (Singh, Junnarkar) | |||||||||
| NFPA 704 |
|
Cobalt chloride price More Price(25)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 8.02540 | Cobalt(II) chloride anhydrous for synthesis | 7646-79-9 | 10G | ₹5959.99 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.02540 | Cobalt(II) chloride anhydrous for synthesis | 7646-79-9 | 25G | ₹9540 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.02540 | Cobalt(II) chloride anhydrous for synthesis | 7646-79-9 | 100G | ₹36610 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 60818 | Cobalt(II) chloride purum p.a., anhydrous, ≥98.0% (KT) | 7646-79-9 | 50G | ₹8183.7 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 60818 | Cobalt(II) chloride purum p.a., anhydrous, ≥98.0% (KT) | 7646-79-9 | 250G | ₹34964.75 | 2022-06-14 | Buy |
Cobalt chloride Chemical Properties,Uses,Production
Description
blue crystals (anhydrous)
violet-blue (dihydrate)
rose red crystals (hexahydrate)
Sinks and mixes with water. Pale blue leaflets, turns pink upon exposure to moist air.
Chemical Properties
(1) Blue, (2) ruby-red crystals.Soluble in water, alcohol, and acetone.
Physical properties
Blue leaflets; turns pink in moist air; hygroscopic; the dihydrate is violet blue crystal; the hexahydrate is pink monoclinic crystal; density 3.36, 2.48 and 1.92 g/cm3 for anhydrous salt, dihydrate and hexahydrate, respectively; anhydrous salt melts at 740°C and vaporizes at 1,049°C; vapor pressure 60 torr at 801°C; the hexahydrate decomposes at 87°C; the anhydrous salt and the hydrates are all soluble in water, ethanol, acetone, and ether; the solubility of hydrates in water is greater than the anhydrous salt.
Uses
Cobalt chloride (CoCl2) is used to manufacture vitamin B12, even though the compound itself can cause damage to red blood cells. It is also used as a dye mordant (to fix the dye to the textile so that it will not run). It is also of use in manufacturing solid lubricants, as an additive to fertilizers, as a chemical reagent in laboratories, and as an absorbent in gas masks, electroplating, and the manufacture of vitamin B12.
Definition
ChEBI: A cobalt salt in which the cobalt metal is in the +2 oxidation state and the counter-anion is chloride. It is used as an indicator for water in desiccants.
General Description
Cobalt(II) chloride is an anhydrous cobalt salt. Cobalt(II) chloride participates in the synthesis of various esters in the presence of acetonitrile.
Air & Water Reactions
Hygroscopic. Soluble in water.
Reactivity Profile
A 0.2 molar aqueous solution has a pH of 4.6. Cobalt chloride acts as a weakly acidic inorganic salt, which is soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. Potassium or sodium metals act to reduce metal halides, producing exothermic reactions, even explosions [Bretherick, 5th Ed., 1995].
Hazard
May not be used in food products (FDA). Can cause blood damage.
Health Hazard
Inhalation causes respiratory disease, shortness of breath, and coughing; permanent disability may occur. Ingestion causes pain, vomiting, and diarrhea. Contact causes irritation of eyes and may cause skin rash.
Fire Hazard
Special Hazards of Combustion Products: Toxic cobalt oxide fumes may form in fire.
Safety Profile
Suspected carcinogen with experimental carcinogenic data. Poison experimentally by ingestion, skin contact, intraperitoneal, intravenous, and subcutaneous routes. Moderately toxic to humans by ingestion. Human systemic effects by ingestion: anorexia, goiter (increased thyroid size), and weight loss. Experimental teratogenic and reproductive effects. Human mutation data reported. Incompatible with metals (e.g., sodmm and potassium). See also COBALT. When heated to decomposition it emits toxic fumes of Cl-.
Purification Methods
A saturated aqueous solution at room temperature is fractionally crystallised by standing overnight. The first half of the material that crystallises in this way is used in the next crystallisation. The process is repeated several times, water being removed in a dry-box using air filtered through glass wool and dried over CaCl2 [Hutchinson J Am Chem Soc 76 1022 1954]. It has also been crystallised from dilute aqueous HCl. The hexahydrate m 86o forms pink to red deliquescent crystals. It loses 4H2O on heating at 52-56o and forms the violet dihydrate which loses a further H2O at 100o to form the violet monohydrate which loses the last H2O at 120-140o to give the pale blue anhydrous deliquescent salt m 735o and b 1049o. A pink solution of CoCl2 in H2O becomes blue on heating to 50o or adding conc HCl which may precipitate the mono or dihydrate. The solid dihydrate gives a blue-purple solution with EtOH. Note: CoCl2 in H2O is a “sympathetic ink”, i.e. writing using an aqueous solution is almost invisible on paper, but becomes blue on warming the paper. On cooling or standing, the writing becomes invisible again. The anhydrous salt is soluble in H2O, EtOH, Et2O, Me2CO and pyridine. [Glemser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1515 1965.]
Cobalt chloride Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of6
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| Evans Fine Chem | +91-9821340302 +91-9821340302 | Maharashtra, India | 286 | 58 | Inquiry |
| Kronox Lab Sciences Pvt Ltd | +91-9313231074 +91-9313231074 | Gujarat, India | 240 | 58 | Inquiry |
| Dhara Industries | +91-9322395199 +91-9322395199 | Mumbai, India | 190 | 58 | Inquiry |
| Parshva Chemicals | +91-7925834748 +91-9825029996 | Gujarat, India | 21 | 58 | Inquiry |
| Anchor Chemicals Industries | +91-9821232090 +91-9819947105 | Maharashtra, India | 16 | 58 | Inquiry |
| Svaks Biotech India Pvt Ltd | +91-7709222999 +91-7767806001 | Maharashtra, India | 81 | 58 | Inquiry |
| Monopoly Innovations Pvt. Ltd. | +undefined-9321425897 +undefined-9819745149 | Maharashtra, India | 45 | 58 | Inquiry |
| Kezi Industries (Sam Industries) | +91-9426862414 +91-9825269675 | Gujarat, India | 34 | 58 | Inquiry |
| S.K. CHEMICAL INDUSTRIES | +91-22-66370299 Ext 21 / 91-22-23437380 Ext 25 | New Delhi, India | 262 | 30 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| Evans Fine Chem | 58 |
| Kronox Lab Sciences Pvt Ltd | 58 |
| Dhara Industries | 58 |
| Parshva Chemicals | 58 |
| Anchor Chemicals Industries | 58 |
| Svaks Biotech India Pvt Ltd | 58 |
| Monopoly Innovations Pvt. Ltd. | 58 |
| Kezi Industries (Sam Industries) | 58 |
| S.K. CHEMICAL INDUSTRIES | 30 |
7646-79-9(Cobalt chloride)Related Search:
1of4
chevron_right




