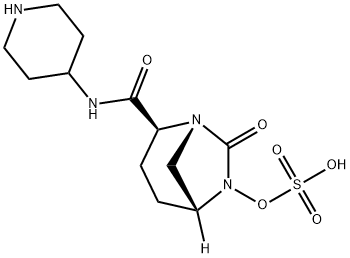
Relebactam
- CAS No.
- 1174018-99-5
- Chemical Name:
- Relebactam
- Synonyms
- MK7655;CS-2637;RELEBACTAM;Relibactam;Ruilai Batan;2H4]-Relebactam;Cefadroxil Monomer;MK-7655(Relebactam);Relebactam(MK-7655);MK-7655;MK 7655;MK7655
- CBNumber:
- CB82546782
- Molecular Formula:
- C12H20N4O6S
- Molecular Weight:
- 348.38
- MOL File:
- 1174018-99-5.mol
- Modify Date:
- 2024/3/29 16:09:00
| Melting point | >252°C (dec.) |
|---|---|
| Density | 1.59 |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Water (Slightly) |
| pka | -4.59±0.18(Predicted) |
| form | Solid |
| color | Off-White to Light Yellow |
| Stability | Hygroscopic |
| InChI | InChI=1S/C12H20N4O6S/c17-11(14-8-3-5-13-6-4-8)10-2-1-9-7-15(10)12(18)16(9)22-23(19,20)21/h8-10,13H,1-7H2,(H,14,17)(H,19,20,21)/t9-,10+/m1/s1 |
| InChIKey | SMOBCLHAZXOKDQ-ZJUUUORDSA-N |
| SMILES | S(O)(ON1C(=O)[N@@]2C[C@@]1([H])CC[C@H]2C(NC1CCNCC1)=O)(=O)=O |
Relebactam Chemical Properties,Uses,Production
Description
Relebactam (formerly known as MK-7655) is a novel,intravenous,class A and class C B-lactamase inhibitor and is currently under evaluation in combination with imipenem/cilastatin for the treatment of resistant Gram-negative infections.In vitro studies demonstrated that relebactam restored imipenem's activity against KPC-producing Enterobacteriacae,lowering imipenem MICs from 16-64 to 0.12-1mg/L at a concentration of 4 mg/L. Moreover, relebactam is able to lower imipenem MICs for P. aeruginosa,particularly in strains with depressed OprD expression and increased AmpC expression.Conversely, the addition of relebactam to imipenem does not seem to provide any adjunctive benefit against A.baumanii or S.maltophilia or against MBL-producing Enterobacteriacae.A non-inferiority, Phase 3 trial evaluating the efficacy and safety of imipenem/relebactam compared to piperacillin/tazobactam for the treatment of HAP and VAP(ClinicalTrials.gov Identifier: NCT02493764) is currently recruiting. A Phase 3 study evaluating the efficacy and safety of imipenem/relebactam (200/100-500/250 mg depending on renal function) compared to colistimethate sodium plus imipenem/cilastatin for the treatment of imipenem-resistant bacterial infections,including HAP, VAP, cIAIs and cUTIs, has recently been completed and results are pending(ClinicalTrials.gov Identifier: NCTO2452047).In Phase 2 trials, imipenem/relebactam was well tolerated, with diarrhea, nausea, vomiting and headache being the most commonly reported adverse events.
Uses
Relebactam is a novel β-lactamase inhibitor in combination with Primaxin. It has also demonstrated the potential to enhance the activity of imipenem against P.aeruginosa, including those strains with depressed OprD expression and increased AmpC expression.
Mechanism of action
Relebactam is a beta-lactamase inhibitor known to inhibit many types of beta-lactamases including Ambler class A and Ambler class C enzymes, helping to prevent imipenem from degrading in the body.Label,Similar to the structurally-related avibactam, first, relebactam binds non-covalently to a beta-lactamase binding site, then, it covalently acylates the serine residue in the active site of the enzyme.In contrast to some other beta-lactamase inhibitors, once relebactam de-acylates from the active site, it can reform it's 5 membered ring and is capable of rebinding to target enzymes.
https://go.drugbank.com/drugs/DB12377
Clinical Use
Recently, another carbapenem-β-lactamase inhibitor, imipenem/cilastatin-relebactam (RecarbrioTM), was approved by the FDA. Relebactam, a bicyclic diazabicyclooctane, is structurally related to avibactam but differs by the addition of a piperidine ring to the 2-position carbonyl group. Like meropenem- vaborbactam (VabomereTM), imipenem-relebactam(RecarbrioTM) is active against class A and class C carbapenemases and is approved for the treatment of multidrug-resistant intraabdominal infections secondary to Bacteroides caccae, Bacteroides fragilis, Bacteroides ovatus, Bacteroides thetaiotaomicron, Bacteroides uniformis, Bacteroides vulgatus, Bifidobacterium stercoris, Citrobacter freundii, Enterobacter cloacae, Escherichia coli, Fusobacterium nucleatum, Klebsiella aerogenes, Klebsiella oxytoca, Klebsiella pneumoniae, Parabacteroides distasonis, and Pseudomonas aeruginosa and multidrug-resistant complicated urinary tract infections secondary to E. cloacae, E. coli, K. aerogenes, K. pneumoniae and P. aeruginosa.
Synthesis
Relebactam is a β-lactamase inhibitor in clinical trials as a combination therapy for the treatment of bacterial infection resistant to β-lactam antibiotics. Its unusual structural challenges have inspired a rapid synthesis featuring an iridium-catalyzed N–H insertion and a series of late stage transformations designed around the reactivity of the labile bicyclo[3.2.1]urea at the core of the target.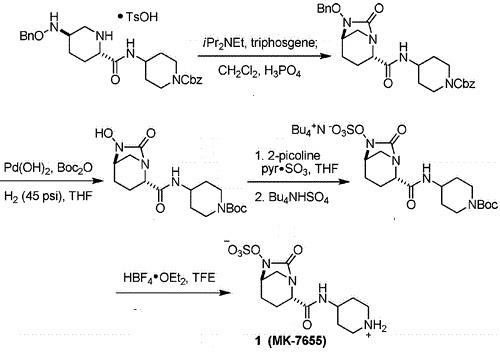
In 2014, Qualified Infectious Disease Product (QIDP) and Fast Track designations were assigned by the FDA for the treatment of complicated urinary tract infections, complicated intra-abdominal infections and hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia.
A Concise Synthesis of a β-Lactamase Inhibitor
Relebactam Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Heading (Nanjing)Pharmtechnologies Co., Ltd. | +86-25-58467899-832 +86-13382406280 | China | 46 | 58 | Inquiry |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | China | 20293 | 58 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32836 | 60 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29888 | 58 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | China | 10522 | 58 | Inquiry |
| ChemExpress | +86-021-58950125 +86-021-58950125; | China | 598 | 58 | Inquiry |
| InvivoChem | +1-708-310-1919 +1-13798911105 | United States | 6393 | 58 | Inquiry |
Related articles
- How to synthesize Relebactam?
- Relebactam, formerly MK-7655, is a DBO that promises to contribute to the renaissance in antimicrobial chemotherapy.
- Jan 5,2024
1174018-99-5(Relebactam)Related Search:
1of4
chevron_right




