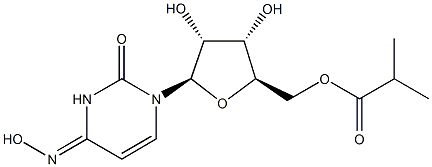
Molnupiravir
- CAS No.
- 2349386-89-4
- Chemical Name:
- Molnupiravir
- Synonyms
- Molnupiravir;EIDD-2801;((2R,3S,4R,5R)-3,4-dihydroxy-5-((E)-4-(hydroxyimino)-2-oxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl isobutyrate;((2R,3S,4R,5R)-3,4-dihydroxy-5-(4-(hydroxyamino)-2-oxopyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl isobutyrate;Tube994;Molnupiravir(MK-4482);Molnupiravir(EIDD-2801);IDD-2801;Mopiravir;EIDO-2801
- CBNumber:
- CB85851964
- Molecular Formula:
- C13H19N3O7
- Molecular Weight:
- 329.31
- MOL File:
- 2349386-89-4.mol
- Modify Date:
- 2024/9/10 19:09:04
| Melting point | 156 - 159°C |
|---|---|
| storage temp. | -20°C, Inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
| InChI | InChI=1/C13H19N3O7/c1-6(2)12(19)22-5-7-9(17)10(18)11(23-7)16-4-3-8(15-21)14-13(16)20/h3-4,6-7,9-11,17-18,21H,5H2,1-2H3,(H,14,15,20)/t7-,9-,10-,11-/s3 |
| InChIKey | HTNPEHXGEKVIHG-JFPRWSRANA-N |
| SMILES | O[C@@H]1[C@@H]([C@@H](COC(=O)C(C)C)O[C@H]1N1C=C/C(=N/O)/NC1=O)O |&1:1,2,3,12,r| |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H372 |
| Precautionary statements | P260-P264-P270-P314-P501 |
Molnupiravir Chemical Properties,Uses,Production
Description
EIDD-2801 is an orally bioavailable prodrug of the antiviral nucleoside derivative N4- hydroxycytidine (NHC, EIDD-1931). It is a nucleotide analog inhibitor of RNA-dependent RNA polymerases (RdRps). The compound interferes with the action of viral RNA polymerase. It exerts its antiviral action through introduction of copying errors during viral RNA replication. The active drug incorporates into the genome of RNA viruses, leading to an accumulation of mutations known as viral error catastrophe. The broadspectrum antiviral agent EIDD-2801 inhibits viral RNA replication in various unrelated RNA viruses including influenza, Ebola, Venezuelan equine encephalitis virus (VEEV) and coronaviruses, including SARS-CoV, MERS-CoV and SARS-CoV-2. EIDD-2801 has the potential for COVID-19, seasonal and pandemic influenza treatment.
History
Molnupiravir (EIDD-2801/MK-4482) is an investigational, orally bioavailable form of a potent ribonucleoside analog that inhibits the replication of multiple RNA viruses including SARS-CoV-2, the causative agent of COVID-19. Molnupiravir has been shown to be active in several models of SARS-CoV-2, including for prophylaxis, treatment and prevention of transmission, as well as SARS-CoV-1 and MERS. EIDD-2801 was invented at Drug Innovations at Emory (DRIVE), LLC, a not-for-prot biotechnology company wholly owned by Emory University, and with partial funding support from the U.S. government. Since licensed by Ridgeback, all funds used for the development of EIDD-2801 by Ridgeback have been provided by Wayne and Wendy Holman and Merck.
Definition
Molnupiravir is a nucleoside analogue that is N(4)-hydroxycytidine in which the 5'-hydroxy group is replaced by a (2-methylpropanoyl)oxy group. It is the prodrug of the active antiviral ribonucleoside analog N(4)-hydroxycytidine (EIDD-1931), has activity against a number of RNA viruses including SARS-CoV-2, MERS-CoV, and seasonal and pandemic influenza viruses. It is currently in phase III trials for the treatment of patients with COVID-19. It has a role as a prodrug, an anticoronaviral agent and an antiviral drug. It is a nucleoside analogue, an isopropyl ester and a ketoxime. It derives from a N(4)-hydroxycytidine.
www.ebi.ac.uk
Biological Activity
EIDD-2801 is an orally active, 5′-isopropylester prodrug form of the antiviral ribonucleoside analog N4-hydroxycytidine (NHC, EIDD-1931) with known anti-viral activity against SARS-CoV, SARS-CoV-2, RSV, influenza A & B (IAV & IBV), HCV, pestivirus, bovine viral diarrhoea virus (BVDV), Ebola (EBOV), Chikungunya (CHIKV), venezuelan equine encephalitis (VEEV). EIDD-2801 is efficiently hydrolyzed to NHC in vivo, being more orally bioavailable than NHC in nonhuman primates and ferrets, while maintaining similar oral bioavailability as NHC in mice. EIDD-2801 displays in vivo efficacy against pandemic and seasonal IAV strains in ferrets (lowest ED = 2.3 and 7 mg/kg b.i.d. p.o., respectively).
Regulatory Status
Molnupiravir (as EIDD-2801) was developed at Drug Innovation Ventures at Emory (DRIVE), a not-forprofit biotechnology company owned by Emory University, Atlanta. It was then licensed by Ridgeback Biotherapeutics, and is now developed further in cooperation with Merck & Co. (in Europe: Merck Sharp & Dohme/ MSD).
Molnupiravir is not approved by the European Medicines Agency (EMA) or the American Food and Drug Administration (FDA).
References
[1] Toots M, Yoon J-J, Cox RM et al. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Science Translational Medicine 2019;11(515). Epub 23 Oct 2019.
[2] Toots M, Yoon J-J, Hart M et al. . Quantitative Efficacy Paradigms of the Influenza Clinical Drug Candidate EIDD-2801 in the Ferret Model. Transl Res 2020. Epub 2019 Dec 25.
[3] Halford B. An emerging antiviral takes aim at COVID-19. American Chemical Society; 2020 [14.12.2020]; Available from: https://cen.acs.org/pharmaceuticals/drug-development/emergingantiviral-takes-aim-COVID-19/98/web/2020/05.
[4] Ridgeback Biotherapeutics. OVERVIEW OF EIDD-2801 (MK-4482). [Presentation]. provided via email 2020.
Molnupiravir Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| Vijayasri Organics Private Limited | +91-9848990113 +91-9848990113 | Telangana, India | 43 | 58 | Inquiry |
| BIOCHEMICAL & SYNTHETIC PRODUCTS PVT. LT., | +91-91-8421973722 +91-9869455944 | Telangana, India | 31 | 58 | Inquiry |
| Bulat Pharmaceutical Pvt Ltd | +91-8448085659 +91-8448085660 | Haryana, India | 123 | 58 | Inquiry |
| Viruj Pharmaceuticals Pvt Ltd | +91-9441591589 +91-9441591589 | Hyderabad, India | 33 | 58 | Inquiry |
| GLP Pharma Standards | +91 9866074638 | Hyderabad, India | 1644 | 58 | Inquiry |
| Gonane Pharma | +91-9819380043 +91-9819380043 | NaviMumbai, India | 192 | 58 | Inquiry |
| Sekhment pharmaventures | +91-9030088669 +91-9030088669 | Mumbai, India | 105 | 58 | Inquiry |
| Synaptics Labs Private Limited | +91-8341963666 +91-8341963666 | Hyderabad, India | 84 | 58 | Inquiry |
| Zenfold Sustainable Technologies (ZST) | +91-9945888778 +91-9945888778 | Karnataka, India | 32 | 58 | Inquiry |
Related articles
- How is Molnupiravir synthesised?
- Molnupiravir is synthesised using uridine as a raw material by chemical reaction.
- Jan 9,2024
- Mechanism and adverse effects of Molnupiravir
- Molnupiravir is a product that the FDA is allowing to be given for emergency use to treat COVID-19.
- Apr 15,2022
- A nucleoside analogue:Molnupiravir
- Molnupiravir(EIDD-2801) is a nucleoside analogue that is N(4)-hydroxycytidine in which the 5'-hydroxy group is replaced by a (....
- Mar 23,2022





