alfentanil
- CAS No.
- 71195-58-9
- Chemical Name:
- alfentanil
- Synonyms
- Rapifen;Hsdb 6789;Alfentanyl;alfentanil;Brn 1188293;Dea no. 9737;Alfentanilum;alfentanil USP/EP/BP;Alfentanilum [inn-latin];70879-28-6 (Hydrochloride)
- CBNumber:
- CB8875635
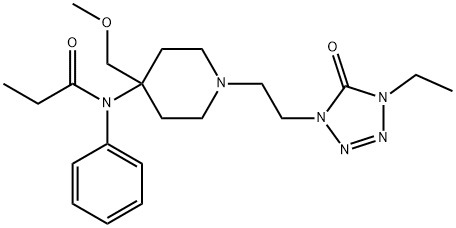
- Molecular Formula:
- C21H32N6O3
- Molecular Weight:
- 416.52
- MOL File:
- 71195-58-9.mol
- Modify Date:
- 2022/12/21 16:56:50
alfentanil Chemical Properties,Uses,Production
Uses
The low pKa of alfentanil (6.9) results in it being largely unionised at plasma pH, allowing rapid diffusion to the effect site (t1.2keo ~1min) and rapid onset of action despite it being less lipid soluble than other opioids. It does not bind strongly to opioid receptors, and the effect-site concentration also decreases rapidly as plasma concentrations decrease. Alfentanil is metabolised by the hepatic cytochrome P450 isoform CYP3A4. Genetic variability in the activity of this enzyme may result in two- to threefold variations in pharmacokinetic values when given by infusion. Low, medium or high metabolisers have been identified; this has implications for duration of action when prolonged use is contemplated.
Definition
ChEBI: A member of the class of piperidines that is piperidine having a 2-(4-ethyl-5-oxo-4,5-dihydro-1H-tetrazol-1-yl)ethyl group at the 1-position as well as N-phenylpropanamido- and methoxymethyl groups at the 4-position.
General Description
The addition of a methoxy methyl on the 4-piperidine and thesubstitution of the phenethyl ring for an ethyl-substitutedtetrazole-one yielded a compound with about one fourth toone third the potency of fentanyl. Although lesspotent, it has a quicker onset of action, a shorter duration ofaction, and thus a better, safety profile for use as an anestheticadjunct. The piperidine amine has a pKa of 6.5 compared withfentanyl’s pKa of 8.4. This results in a higher proportion ofunionized drug for alfentanil leading to quicker penetrationthrough the blood-brain barrier and thus onset of action.Alfentanil (Alfenta) is available as an IV injection for use asan analgesic adjunct for induction of general anesthesia and tomaintain analgesia during general surgical procedures.





