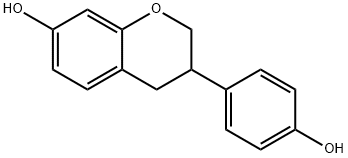
(+/-)-EQUOL
- CAS No.
- 94105-90-5
- Chemical Name:
- (+/-)-EQUOL
- Synonyms
- NV 07α;(+/-)Equol;(+/-)-EQUOL;Equol,99%e.e.;EQUOL(RACEMIC);(+/-)-Equol>(+/-)-Equol (GMP);4',7-Isoflavandiol;7,4'-HoMoisoflavane;(±)-EQUOL >= 99.0% (TLC)
- CBNumber:
- CB8972097
- Molecular Formula:
- C15H14O3
- Molecular Weight:
- 242.27
- MOL File:
- 94105-90-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/22 14:21:17
| Melting point | 158-160?C |
|---|---|
| Boiling point | 441.7±45.0 °C(Predicted) |
| Density | 1.286±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | soluble in Ethanol |
| form | Crystalline powder |
| pka | 9.94±0.40(Predicted) |
| color | White to Light yellow to Light red |
| Water Solubility | Soluble in DMSO and methanol or 100% ethanol. Insoluble in water. |
| Sensitive | Hygroscopic |
| BRN | 87752 |
| InChI | InChI=1S/C15H14O3/c16-13-4-1-10(2-5-13)12-7-11-3-6-14(17)8-15(11)18-9-12/h1-6,8,12,16-17H,7,9H2 |
| InChIKey | ADFCQWZHKCXPAJ-UHFFFAOYSA-N |
| SMILES | C1OC2=CC(O)=CC=C2CC1C1=CC=C(O)C=C1 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H332-H335 |
| Precautionary statements | P261-P280-P305+P351+P338 |
| WGK Germany | 3 |
| HS Code | 2932990090 |
(+/-)-EQUOL price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 45405 | (±)-Equol ≥99.0% (TLC) | 94105-90-5 | 1MG | ₹26781.05 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 45405 | (±)-Equol ≥99.0% (TLC) | 94105-90-5 | 5MG | ₹88678.4 | 2022-06-14 | Buy |
| TCI Chemicals (India) | E0922 | (±)-Equol min. 98.0 % | 94105-90-5 | 200MG | ₹8800 | 2022-05-26 | Buy |
(+/-)-EQUOL Chemical Properties,Uses,Production
Description
Equol is a nonsteroidal estrogen produced from the metabolism of the isoflavonoid phytoestrogen daidzein by human intestinal microflora. The estrogen receptor (ER) binding activity of the naturally occurring (S)-enantiomer demonstrates greater affinity toward ERβ while the (R)-enantiomer demonstrates greater affinity towards ERα. Synthesized as a racemic mixture, (±)-equol exhibits EC50 values of 200 and 74 nM for human ERα and ERβ, respectively and induces breast cancer cell proliferation in vitro at concentrations as low as 100 nM.
Chemical Properties
White to Off-White Solid
Uses
(+/-)-Equol exhibits EC50 values of 200 and 74 nM for human ERα and ERβ, respectively and induces breast cancer cell proliferation in vitro at concentrations as low as 100 nM. It is a weak estrogenic agonist, and competitive inhibitor of 17β-estradiol at the estradiol receptor. It inhibits 12-O-tetradecanoylphorbol 13-acetate (TPA)-induced neoplastic cell transformation by targeting the MEK/ERK/p90RSK/activator protein-1 signalling pathway. It functions as a DHT blocker.
Application
(±)-Equol is a metabolite produced in vivo from the soy phytoestrogen daidzein by the action of certain gut microflora, and is a nonsteroidal estrogen of the isoflavonoid class. (±)-Equo has been reported to have estrogenic activity and affinity for estrogen receptors. (±)-Equo can exist in two enantiomeric forms, (S)-equol and (R)-equol. In binding assays, (S)-equol has a higher binding affinity for estrogen β receptors.
Biological Activity
Metabolite of daidzein. Weak estrogenic agonist, and competitive inhibitor of 17β-estradiol at the estradiol receptor.
(R,S)-Equol is a flavonoid. Racemic mixture. This is a metabolite that is produced in vivo from soy phytoestrogen daidzein from gut microflora. (R,S)-Equol inhibits 12-O-tetradecanoylphorbol 13-acetate (TPA)-induced neoplastic cell transformation by targeting the MEK/ERK/p90RSK/activator protein-1 signalling pathway. (R,S)-Equol shows positive effects on the incidence of prostate cancer. Functions as a DHT blocker. Preferentially activates estrogen receptor β (ERβ).
References
Equol, a natural estrogenic metabolite from soy isoflavones convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta
R. S. Muthyala, Y. H. Ju, S. Sheng, L. D. Williams, D. R. Doerge, B. S. Katzenellenbogen, W. G. Helferich, J. A. Katzenellenbogen, Bioorg. Med. Chem. 2004, 12, 1559.
Isolation and identification of new bacterial stains producing equol from Pueraria lobate extract fermentation
J. E. Kwon, J. Lim, I. Kim, D. Kim, S. C. Kang, PLoS ONE 2018, 13, e0192490.
Setchell, K.D.R., Clerici, C., Lephart, E.D., et al. S-equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 81(5), 1072-1079 (2005).
Liu, H., Du, J., Hu, C., et al. Delayed activation of extracellular-signal-regulated kinase ? is involved in genistein- and equol-induced cell proliferation and estrogen-receptor-α-mediated transcription in MCF-7 breast cancer cells. Journal of Nutritional Biochemistry (2009).
(+/-)-EQUOL Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Gagri Global IT Services Pvt. Ltd. | +91-40-27170174, | Telangana, India | 1321 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Hangzhou Weitai Biological Pharmaceutical Co.,Ltd. | +8613282012786 | China | 61 | 58 | Inquiry |
| Wuhan Nutra Biotechnology Co.,Ltd | +8617786394783 | China | 300 | 58 | Inquiry |
| Qingdao Yonghefeng Economic and Trade Co., Ltd. | +86-13375559818 +86-13375559818 | China | 195 | 58 | Inquiry |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | China | 5895 | 58 | Inquiry |
| Chongqing Zhihe Biopharmaceutical Co., Ltd. | +8618580541567 | China | 303 | 58 | Inquiry |





