Barium hypochlorite
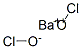
- CAS No.
- 13477-10-6
- Chemical Name:
- Barium hypochlorite
- Synonyms
- Barium hypochlorite.;Dihypochlorous acid barium salt
- CBNumber:
- CB91245139
- Molecular Formula:
- Ba.2ClHO
- Molecular Weight:
- 242.25
- MOL File:
- 13477-10-6.mol
- Modify Date:
- 2024/3/14 15:18:29
| EPA Substance Registry System | Hypochlorous acid, barium salt (13477-10-6) |
|---|
Barium hypochlorite Chemical Properties,Uses,Production
Physical properties
Barium hypochlorite has the formula, BaClO2 and the molecular weight of 240.234 g/mol. Its CAS number is 13477-10-6. It is white-colored crystalline solid that is denser than water. Contact may irritate skin, eyes and mucous membranes. It may be toxic by ingestion. It will accelerate the burning of combustible materials. Prolonged exposure to heat or flames may result in an explosion. It reacts with acids, evolving chlorine, an irritating, corrosive and toxic gas. It reacts fiercely with cyanides when heated or by friction. It may form explosive mixtures with combustible material, powdered metals or ammonium compounds. It is not offered commercially for sale nor is the scientific literature concerning its uses or physical and chemical properties readily available.
General Description
A white-colored crystalline solid. Denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. May accelerate the burning of combustible materials. Prolonged exposure to heat or flames may result in an explosion.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Salts of hypochlorous acid, HClO. Generally toxic, irritants and powerful oxidizers, particularly in the presence of water or at higher temperature as they decompose to release oxygen and chlorine gases. On contact with urea they form the highly explosive NCl3 . When heated or on contact with acids, they produce highly toxic fumes of chlorine gas [Sax, 9th ed., 1996, p. 1905].
Health Hazard
Toxic by ingestion. Inhalation of dust is toxic. Fire may produce irritating, corrosive and/or toxic gases. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. May explode from heat or contamination. Some may burn rapidly. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Barium hypochlorite Preparation Products And Raw materials
Raw materials
Preparation Products
Barium hypochlorite Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| HONEST JOY HOLDINGS LIMITED | +86-755-26404303 | United States | 6702 | 54 | Inquiry |
| Supplier | Advantage |
|---|---|
| HONEST JOY HOLDINGS LIMITED | 54 |





