URANYL NITRATE
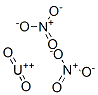
- CAS No.
- 10102-06-4
- Chemical Name:
- URANYL NITRATE
- Synonyms
- D014502;UO2(NO3)2;URANYL NITRATE;Nitrate, uranyl;Uranyl dinitrate;Nitrate, uranium;URANYL(2+)NITRATE,SOLID;Uranium, bis(nitrato-o)dioxo-, (T-4)-;Uranyl nitrate (solid): (Bis(nitrato-O,O')dioxouranium)
- CBNumber:
- CB9257520
- Molecular Formula:
- N2O8U
- Molecular Weight:
- 394.04
- MOL File:
- 10102-06-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/18 15:15:00
| Melting point | 60.2°C |
|---|---|
| Boiling point | 118°C |
| Density | 2.807 |
| solubility | soluble in ethyl ether |
| form | yellow-green solid |
| color | yellow crystals, crystalline |
| Water Solubility | 127 at 25℃ |
| EPA Substance Registry System | Uranyl nitrate (10102-06-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS08,GHS09,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H411-H373-H330-H300 | |||||||||
| Precautionary statements | P260-P314-P501-P264-P270-P301+P310-P321-P330-P405-P501-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501 | |||||||||
| NFPA 704 |
|
URANYL NITRATE Chemical Properties,Uses,Production
Chemical Properties
Yellow, rhombic crystals. Soluble in water, alcohol, and ether.
Physical properties
The hexahydrate is a yellow crystalline solid; orthogonal crystals; density 2.81 g/cm3; hygroscopic; melts at 60°C; decomposes at 118°C; very soluble in water; soluble in alcohol and ether.
Uses
Uranyl nitrate is a yellow poisonous crystal made by dissolving
uranic oxide in nitric acid. It is soluble in water, alcohol, and
ether. First suggested by C. J. Burnett in 1855, a uranyl nitrate
printing process was patented by Niépce de St. Victor in 1858.
The paper was floated on a uranyl nitrate solution and dried
in the dark. The paper was exposed under a negative in the
sun until a faint image appeared. The image was developed to
completion by floating the paper on a solution of silver nitrate
or gold chloride followed by washing. A process patented in
1864 by Jacob Wothly called for adding uranyl nitrate and
silver to collodion, which was then applied to paper. The
Wothlytype was a printing-out process.
Uranyl nitrate was used in printing-out emulsions and with
potassium ferricyanide for toning prints and intensifying negatives.
It was also used with silver nitrate for making uranium/
silver gaslight papers.
Preparation
Uranyl nitrate is obtained as an intermediate in recovering uranium from its minerals. The compound can be prepared by reacting triuranium octaoxide, U3O8, with nitric acid. It is separated and purified by extraction with ether.
Hazard
Strong oxidizer. Corrosive and irritating.
Safety Profile
Poison by inhalation. Moderately toxic by ingestion. Human mutation data reported. A corrosive irritant to skin, eyes, and mucous membranes. A radioactive material. A powerful explosive and oxibzer. Incompatible with cellulose. Ether solutions in sunlight may explode. When heated to decomposition it emits toxic fumes of NOx. See also URANYL NITRATE HEXAHYDRATE and URANIUM.
URANYL NITRATE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21669 | 55 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 9003 | 58 | Inquiry |
| Henan Lien Chemical Products Co., Ltd. | 15378766539 | China | 4017 | 58 | Inquiry |
| Aithaca Chemical Corp. | (516) 229-2330 | United States | 1684 | 30 | Inquiry |
| ProChem, Inc. | 815 398 1788 | United States | 1692 | 66 | Inquiry |
| Portail Substances Chimiques | 10 20 0000 | France | 6027 | 58 | Inquiry |
| Shaanxi Dideu Newmaterial Co., Ltd. | 029-63373950 15353716720 | China | 10011 | 58 | Inquiry |





