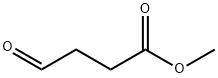
Methyl 4-oxobutanoate
- CAS No.
- 13865-19-5
- Chemical Name:
- Methyl 4-oxobutanoate
- Synonyms
- Methyl 3-Formylpropionate;Einecs 237-611-9;methyl 4-oxobutyrate;METHYL 4-OXOBUTANOATE;Beraprost Impurity 47;Beraprost Impurity 36;Methyl 4-oxobutanoate 90%;Methyl 4-oxobutanoate (~85%);4-Oxobutyric acid methyl ester;4-OXOBUTANOIC ACID METHYL ESTER
- CBNumber:
- CB9383373
- Molecular Formula:
- C5H8O3
- Molecular Weight:
- 116.12
- MOL File:
- 13865-19-5.mol
- Modify Date:
- 2023/12/4 8:57:40
| Boiling point | 187-188 °C(lit.) |
|---|---|
| Density | 1.109 g/mL at 25 °C(lit.) |
| refractive index |
n |
| Flash point | 187 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Oil |
| color | Colourless |
| InChIKey | DLZVZNAPRCRXEG-UHFFFAOYSA-N |
| LogP | 0.110 (est) |
Methyl 4-oxobutanoate price More Price(2)
Methyl 4-oxobutanoate Chemical Properties,Uses,Production
Description
Methyl-4-oxobutyrate is a kind of oxobutyrate derivative. It is an important industrial intermediate. It, together with its methanol addition products such as methyl 4,4-dimethoxybutyrate and gamma- methoxy-gamma-butyrolactone are useful intermediates during the preparation of some important industrial compounds such as gamma- butyrolactone, 1,4-butanediol and glutamate. It is also useful as a model substrate during exploring the asymmetric henry reaction since it can assemble a stereodefined lactam with high efficiency and enantiomeric purity.
Uses
Methyl 4-oxobutanoate may be used as a starting material to synthesize (-)-deoxoprosophylline, a piperidine alkaloid with therapeutic potential.
References
Gresham, William F, R. E. Brooks, and W. M. Bruner. "Preparation of methyl 4-oxobutyrate." US2549454. 1951.
Murib, Jawad H, and W. D. Baugh. "Process for the preparation of methyl 4-oxobutyrate and its methanol addition products." US, US5072005. 1991.
https://www.scbt.com/scbt/product/methyl-4-oxobutanoate-13865-19-5
Methyl 4-oxobutanoate Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6707 | 30 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | China | 29798 | 60 | Inquiry |
| Nextpeptide Inc | +86-0571-81612335 +8613336028439 | China | 19915 | 58 | Inquiry |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | China | 1807 | 55 | Inquiry |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | China | 9337 | 55 | Inquiry |
| Zhejiang ZETian Fine Chemicals Co. LTD | +8618957127338 | China | 2141 | 58 | Inquiry |
| Shanghai Worldyang Chemical Co.,Ltd. | +86-021-56795779 +8613651600618 | China | 1080 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
Related articles
- Methyl 4-oxobutanoate: properties, applications and safety
- Methyl 4-oxobutanoate is a versatile compound with solubility, reactivity, and biological effects, but requires careful handli....
- Dec 4,2023
- Activity and Synthesis of Methyl 4-oxobutanoate
- As a composition, methyl 4-oxobutanoate may be the active unit of some natural products.
- Oct 28,2019
13865-19-5(Methyl 4-oxobutanoate)Related Search:
1of4
chevron_right





