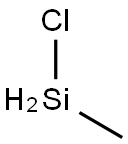
Chloromethyl silane
- CAS No.
- 993-00-0
- Chemical Name:
- Chloromethyl silane
- Synonyms
- Un2534;Einecs 213-600-4;Methylchlorosilane;Chloromethyl silane;Silane, chloromethyl-;Methylchlorosilane,mixed monomer;Methylchlorosilane Mixed Monomers;Silane, chloromethyl-(6CI,7CI,8CI,9CI);Chloromethyl silane ISO 9001:2015 REACH;Methylchlorosilane [un2534] [poison gas]
- CBNumber:
- CB9852514
- Molecular Formula:
- CH5ClSi
- Molecular Weight:
- 80.59
- MOL File:
- 993-00-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/14 15:18:33
| Melting point | -134.1°C |
|---|---|
| Boiling point | 8.7°C (estimate) |
| Density | 0.8840 |
| form | liquid |
| EPA Substance Registry System | Methylchlorosilane (993-00-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS04 |
|---|---|
| Signal word | Danger |
| Hazard statements | H261-H314-H220-H280 |
| Precautionary statements | P231+P232-P280-P370+P378-P402+P404-P501-P210-P377-P381-P403-P410+P403-P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501 |
| RIDADR | 2534 |
| HazardClass | 2.3 |
Chloromethyl silane Chemical Properties,Uses,Production
Definition
One of several intermediates in the formation of silicones or siloxanes, they react with hydroxyl groups on many types of surfaces to produce a permanent, thin-surface film of silicone that imparts water repellency. Examples are methyltrichlorosilane, dimethyldichlorosilane, and trimethylchlorosilane.
General Description
Methyl chlorosilane is a colorless gas with a distinctive odor. Chloromethyl silane is insoluble in water. Its vapors are heavier than air. Contact with the material causes severe irritation to skin, eyes, and mucous membranes. Chloromethyl silane is toxic by ingestion and skin absorption. Chloromethyl silane is also a dangerous fire risk. Chloromethyl silane is used to make water repellent materials. Prolonged exposure of container to fire or intense heat may result in their violent rupturing and rocketing.
Air & Water Reactions
Highly flammable. Based on the properties of similar materials, there is the possibility that the reaction of Chloromethyl silane with water may be vigorous or violent. Products of the reaction include hydrogen chloride. The reaction generates heat and this heat may be sufficient to ignite the product. The chlorosilicon hydrides (ClxSiHy) are spontaneously flammable in air [NFPA 1991].
Reactivity Profile
Chlorosilanes, such as Chloromethyl silane, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases.
Hazard
Toxic by ingestion and inhalation, strong irritant to skin and eyes.
Health Hazard
TOXIC; may be fatal if inhaled or absorbed through skin. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Flammable; may be ignited by heat, sparks or flames. May form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. Vapors may travel to source of ignition and flash back. Some of these materials may react violently with water. Cylinders exposed to fire may vent and release toxic and flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket. Runoff may create fire or explosion hazard.
Chloromethyl silane Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 23699 | 58 | Inquiry |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | China | 52861 | 58 | Inquiry |
| PT CHEM GROUP LIMITED | China | 35426 | 58 | Inquiry | |
| Zhengzhou Acme Chemical Co., Ltd. | 0371-0371-63315541 13323832564 | China | 9914 | 58 | Inquiry |
| WUHAN JIXINYIBANG BIOTECHNOLOGY .,LTD | 027-59768661 13297943853 | China | 5006 | 58 | Inquiry |
| Pand (Shanghai) international trade Co., LTD | 18662931292 18662931292 | China | 4598 | 58 | Inquiry |
| Jiangsu Raien Environmental Protection Technology Co., Ltd | 0513-66814573 16651336298 | China | 2663 | 58 | Inquiry |
| Portail Substances Chimiques | 10 20 0000 | France | 6027 | 58 | Inquiry |
| Central China Special Gas (CCSG) Co., Ltd | 0734-8755555 15674722888 | China | 282 | 58 | Inquiry |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 15229059051 | China | 9977 | 58 | Inquiry |
993-00-0(Chloromethyl silane)Related Search:
1of4
chevron_right




