IBANDRONATE 化学特性,用途語,生産方法
説明
This bisphosphonate, a calcium metabolic inhibitor and
osteogenesis inhibitor, was developed and launched by
Boehringer Mannheim (now Roches) for the treatment of
tumor-induced hypercalcemia (TIH) and is available in both injectable and oral formulations. In In collaboration with
GlaxoSmithKline, the ibandronic acid was also developed in
both iv and oral formulations for the treatment and
prevention of postmenopausal osteoporosis.
合成
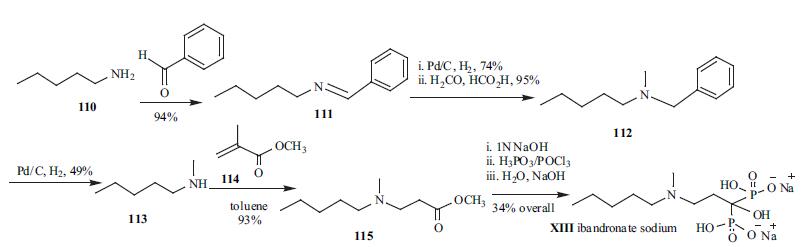
The synthesis of
ibandronate sodium (XIII) is shown in the scheme.
However some reaction details are not available in the
literature. N -pentylamine (110) was reacted with
benzaldehyde to give oily Schiff base 111 in 94% yield.
Hydrogenation with palladium/charcoal gave N-benzyl-Npentylamine
as oil in 74% yield. The secondary amine was
reductively alkylated with formaldehyde and formic acid to
give the tertiary amine 112 in 95% yield. Hydrogenolytic
cleavage of the benzyl group of 112 with palladium/charcoal
gave secondary amine 113, which was reacted with methyl
acrylate (114) in toluene to give compound 115 in 93%
yield. Methyl ester 115 was then saponified with 1N NaOH
to give carboxylic acid. The acid was then heated to 80oC
with phosphorous acid. The melt was mixed with
phosphorus oxychloride at the same temperature for 16
hours. Water was then added and the reaction mixture was
stirred at 100°C for 24 hours to give free diphosphonic acid.
The free diphosphonic acid was finally treated with sodium
hydroxide to give ibandronate sodium (XIII).
IBANDRONATE 上流と下流の製品情報
原材料
準備製品