チタノセンジクロリド 化学特性,用途語,生産方法
外観
赤色~赤褐色, 結晶~粉末
溶解性
クロロホルムに溶け、水で分解する。
特徴
Tiはひずんだ四面体形結合,∠CpTiCp 130.9,131.0˚,Ti-Cl 236.1~236.7 pm,Ti-C 232.6~239.4 pm
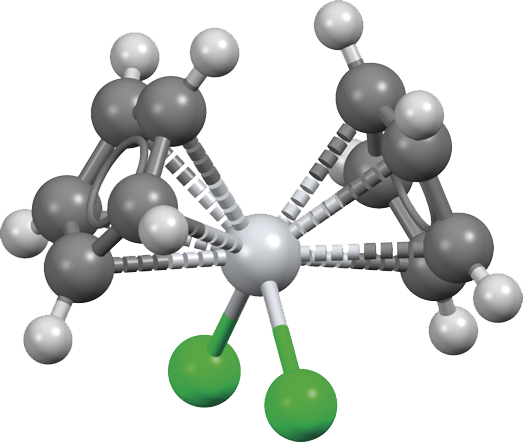
説明
Titanocene dichloride is an organotitanium compound with the chemical formula (η5-C5H5)2TiCl2, often written as Cp2TiCl2, which is a common reagent in organometallic chemistry and organic synthesis. Cp2TiCl2 does not form a "sandwich" structure like ferrocene, but a tetrahedral structure due to its four ligands around a metal center. Because of its anti-tumor activity, it has been used in clinical trials as a chemotherapeutic agent.

化学的特性
Titanocene dichloride is a reddish-orange crystalline solid. Moderately soluble in toluene, chloroform, alcohol, and other hydroxylic solvents; sparingly soluble in water, petroleum ether, benzene, ether, carbon disulfide, and carbon tetrachloride. Stable in dry air, slowly hydrolyzed in moist air. Titanocene dichloride is irritating to the skin and mucous membranes.
使用
Titanocene dichloride is used as an experimental cancer chemotherapeutic agent. It is used as a research chemical, as a catalyst in Ziegler–Natta polymerization reactions, and as an implant material in orthopedics, oral surgery, and neurosurgery.This metallocene is a common reagent in organometallic and organic synthesis. Titanocene dichloride is used to prepare titanocene pentasulfide. Used as an anticancer drug.
調製方法
Titanocene dichloride is produced by the reaction of titanium
tetrachloride with cyclopentadienyl sodium.
一般的な説明
Titanocene dichloride appears as red to red-orange crystals. (NTP, 1992)
空気と水の反応
Stable in dry air. Decomposes in moist air and in water to form HCl .
反応プロフィール
Titanocene dichloride is incompatible with strong oxidizers. Titanocene dichloride may decompose on exposure to water. .
危険性
Toxic by inhalation, irritant to skin and
mucous membranes.
火災危険
Flash point data for Titanocene dichloride are not available; however, Titanocene dichloride is probably combustible.
安全性プロファイル
Poison by intravenous
and intraperitoneal routes. Questionable
carcinogen with experimental neoplastigenic,
tumorigenic, and teratogenic data. Mutation
data reported. See also TITANIUM
COMPOUNDS. When heated to
decomposition it emits toxic fumes of Cl-
純化方法
It forms bright red crystals from toluene or xylene/CHCl3 (1:1) and sublimes at 190o/2mm. It is moderately soluble in EtOH and insoluble in Et2O, *C6H6, CS2, CCl4, pet ether and H2O. The crystalline dipicrate explodes on melting at 139-140o. [Wilkinson et al. J Am Chem Soc 75 1011 1953, IR: Wilkinson & Birmingham J Am Chem Soc 76 4281 1954, NMR and X-ray: Glivicky & McCowan Can J Chem 51 2609 1973, Clearfield et al. J Am Chem Soc 53 1622 1975, Beilstein 16 IV 1769.]
参考文献
A. Clearfield, et al., Can. J. Chem., 53, 1622 (1975), DOI: 10.1139/v75-228.
チタノセンジクロリド 上流と下流の製品情報
原材料
準備製品