テクネチウム 化学特性,用途語,生産方法
解説
テクネチウム,Tc.原子番号43の元素.人工放射性元素.電子配置[Kr]4d55s2の周期表7族第5周期遷移元素.テクネチウムの同位体は現在,質量数85~118まで知られている.D.I. Mendeleev(メンデレーエフ)がエカマンガンとして予言していた元素.1925年にW. Noddack(ノダック)とI. Tackeがウラン鉱物中にX線蛍光スペクトルから43番元素を発見したと報告し,ノダックの故郷の地名Masuriaにちなんでマスリウムと命名したが受け入れられず,1937年にC. PerrierとE. Segréがカリフォルニア大学バークレー校のサイクロトロンで,加速した重陽子でモリブデンを衝撃して合成したものが,確認された最初の43番元素.その後も地球上のTcの探索が続けられ,1962年に至り,P.K. Kuroda(黒田和夫)とB.T. Kennaがアフリカ·ガボン産のせんウラン鉱中に,ウランの自発核分裂生成物として微量の存在を確認した.S,M,N型星にもスペクトル線から存在が推定される.最近,ノダックらのX線スペクトルを詳細に再検討したところ,かれらもTcを見つけていたことが判明した.はじめて人工的につくられた元素として,Segréたちが“人造の”を意味するギリシア語τεχνητο "(technètos)からtechnetiumと命名した.99Tc は,半減期2.11×105 y の比較的安定な β- 崩壊核種で,原子炉使用済みウラン燃料中に核分裂生成物として多量に存在する.現在,医学の分野で広く利用されている 99mTc は,半減期6.0058 h で0.143 MeV のγ線を放出する核種で,99Mo の娘核種として得られる.半減期61 d の 95mTc もトレーサーとして使用される.銀灰色の六方最密金属.融点2172 ℃,沸点4877 ℃.密度11.5 g cm-3(20 ℃ 計算値).7.8 K 以下で超伝導を示す.第一イオン化エネルギー7.28 eV.金属は過テクネチウム酸アンモニウムの水素による還元で得られる.硝酸,王水,濃硫酸に可溶,塩酸に不溶.酸化数2~7.7がもっとも安定で過テクネチウム酸(テトラオキソテクネチウム(Ⅶ)酸イオン)TcO4-として種々の塩をつくる.フッ化物TcF5,TcF6,塩化物TcCl4,TcCl6,臭化物TcBr4などが知られる.酸化物中Tc2O7は融点119.5 ℃,沸点310.6 ℃ でかなり揮発性である.
"(technètos)からtechnetiumと命名した.99Tc は,半減期2.11×105 y の比較的安定な β- 崩壊核種で,原子炉使用済みウラン燃料中に核分裂生成物として多量に存在する.現在,医学の分野で広く利用されている 99mTc は,半減期6.0058 h で0.143 MeV のγ線を放出する核種で,99Mo の娘核種として得られる.半減期61 d の 95mTc もトレーサーとして使用される.銀灰色の六方最密金属.融点2172 ℃,沸点4877 ℃.密度11.5 g cm-3(20 ℃ 計算値).7.8 K 以下で超伝導を示す.第一イオン化エネルギー7.28 eV.金属は過テクネチウム酸アンモニウムの水素による還元で得られる.硝酸,王水,濃硫酸に可溶,塩酸に不溶.酸化数2~7.7がもっとも安定で過テクネチウム酸(テトラオキソテクネチウム(Ⅶ)酸イオン)TcO4-として種々の塩をつくる.フッ化物TcF5,TcF6,塩化物TcCl4,TcCl6,臭化物TcBr4などが知られる.酸化物中Tc2O7は融点119.5 ℃,沸点310.6 ℃ でかなり揮発性である.
化学的特性
closed-packed hexagonal, a=0.2741 nm, c=0.4399nm; enthalpy of sublimation 650 kJ/mol; enthalpy of vaporization ~577 kJ/mol; enthalpy of fusion 33.29 kJ/mol; slowly tarnishes in moist air; when obtained from H2 reduction of ammonium pertechnate, has silvery gray color, and a spongy mass; resembles rhenium in chemical behavior; Debye constant 455K; used as a metallurgical tracer, in nuclear medicine, and to protect against corrosion [HAW93] [MER06] [RAR83] [CRC10]
物理的性質
As the central member of the triad of metals in group 7, technetium (period 5) has similarphysical and chemical properties as its partners manganese (period 4) above it and rhenium(period 6) below it. The sizes of their atomic radii do not vary greatly: Mn = 127, Tc = 136,and Re = 137. Neither does their level of electronegativity vary significantly: Mn = 1.5, Tc =1.9, and Re = 1.9.
Technetium metal is grayish-silver and looks much like platinum. As with most transitionelements, technetium in pure form is a noncorrosive metal. It requires only 55 ppm of technetiumadded to iron to transform the iron into a noncorroding alloy. Because of technetium’sradioactivity, its use as an alloy metal for iron is limited so as to not expose humans to unnecessaryradiation.
Technetium’s melting point is 2,172°C, its boiling point is 4,877°C, and its density is11.50 g/cm
3 .
同位体
There are 47 isotopes. None are stable and all are radioactive. Most are producedartificially in cyclotrons (particle accelerators) and nuclear reactors. The atomicmass of its isotopes ranges from Tc-85 to Tc-118. Most of technetium’s radioactiveisotopes have very short half-lives. The two natural radioisotopes with the longest halflives—Tc-98 = 4.2×10
+6 years and Tc-99 = 2.111×10
+5 years—are used to establishtechnetium’s atomic weight.
名前の由来
Technetium’s name was derived from the Greek word technetos,
meaning “artificial.”
天然物の起源
Technetium is the 76th most abundant element, but it is so rare that it is not found as astable element on Earth. All of it is artificially produced. Even though natural technetium isso scarce that it is considered not to exist on Earth, it has been identified in the light spectrumfrom stars. Using a spectroscope that produces unique lines for each element, scientists areable to view several types of stars. The resulting spectrographs indicate that technetium existsin the stars and thus the universe, but not on Earth as a stable element.
It was the first new element to be produced artificially from another element experimentallyin a laboratory. Today, all technetium is produced mostly in the nuclear reactors of electricalgeneration power plants. Molybdenum-98 is bombarded with neutrons, which then becomesmolybdenum-99 when it captures a neutron. Since Mo-99 has a short half-life of about 66hours, it decays into Tc-99 by beta decay.
特性
Technetium was the first element, not found on Earth, to be artificially produced by bombardingmolybdenum with deuterons.
The major characteristic of technetium is that it is the only element within the 29 transitionmetal-to-nonmetal elements that is artificially produced as a uranium-fission product innuclear power plants. It is also the lightest (in atomic weight) of all elements with no stableisotopes. Since all of technetium’s isotopes emit harmful radiation, they are stored for sometime before being processed by solvent extraction and ion-exchange techniques. The two longlivedradioactive isotopes, Tc-98 and Tc-99, are relatively safe to handle in a well-equippedlaboratory.
Since all of technetium’s isotopes are produced artificially, the element’s atomic weight(atomic mass units) is determined by which isotopes are selected for the calculation.
使用
Technetium is one of the few artificially produced elements that has practical industrial applications.One is that a very small amount (55-ppm) added to iron creates a corrosion-resistantalloy metal. This property is shared with many of the other transition metallic elements, but notwith other artificially produced elements that have higher atomic numbers and are radioactive.
A radioisotope of technetium is widely used in nuclear medicine. The patient is injectedwith saline solution containing Tc-99
m (the superscript “m” means that the isotope is unstableand that its nuclei holds more energy than the regular Tc-99 nuclei into which it decays). Thismeans that the Tc-99
m will start to emit energy and will finally decay and change to the regularnuclei of Tc-99 when injected into the patient. This energy is in the form of very penetratinggamma rays (a strong type of X-rays). The radioactive solution of Tc-99
m may be combinedwith other elements that are absorbed by certain organs of the human body being diagnosedor treated. For instance, adding tin to the solution targets the red blood cells, whereas phosphorusin the solution concentrates the radioactive solution in heat muscles. The gamma raysare strong enough to expose an X-ray film that depicts the internal image of the organ underexamination. This procedure is safe because Tc-99
m has a half-life of only 6.015 hours, andthe Tc-99 has a half-life of over 200,000 years. However, the radioactivity will be harmless inless than a day because the body rapidly eliminates the residual radioactive solution.
Technetium is also used as an alloy metal to produce super-strong magnets that are supercooledto near absolute zero to improve their efficiency. Powerful magnets are used in imagingequipment and possibly in future magnetic driven trains. Its radioactivity makes it useful as atracer in the production of metals and tracing flowing fluids in pipelines.
定義
technetium: Symbol Tc. A radioactivemetallic transition element;a.n. 43; m.p. 2172°C; b.p. 4877°C. Theelement can be detected in certainstars and is present in the fissionproducts of uranium. It was firstmade by Carlo Perrier and EmilioSegré (1905–89) by bombardingmolybdenum with deuterons to givetechnetium–97. The most stable isotopeis technetium–99 (half-life 2.6 ×10
6 years); this is used to some extentin labelling for medical diagnosis.There are sixteen known isotopes.Chemically, the metal has propertiesintermediate between manganeseand rhenium.
製造方法
Technetium isotopes are prepared by bombardment of molybdenum with protons and neutrons. A few nuclear reactions are shown for the three longlived isotopes:
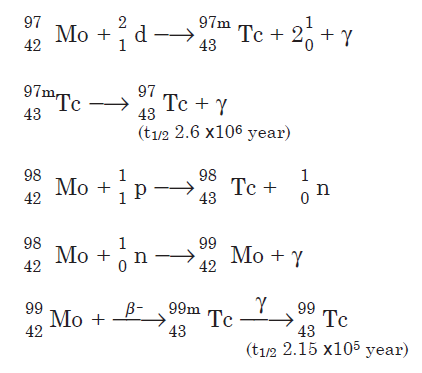
Technetium-99 also is a fission product of uranium-235.
Pure technetium metal may be prepared by reducing ammonium pertechnate, NH4TcO4, with hydrogen at high temperatures. Hydrogen reduction at about 200°C first forms the oxide, TcO
2, which is reduced to Tc metal at 600 to 800°C.
危険性
The hazards of technetium are the same as for all radioactive elements. Excessive exposureto radiation can cause many kinds of tissue damage—from sunburn to radiation poisoningto death.
テクネチウム 上流と下流の製品情報
原材料
準備製品