アイサブコナゾニウム 化学特性,用途語,生産方法
効能
抗真菌薬
説明
Isavuconazonium
sulfate is a broad spectrum antifungal agent that was
codeveloped by Basilea Pharmaceutica (a subsidiary of
Hoffmann-La Roche acquired in 2000) and Astellas Pharma,
which obtained its first approval by the United States Food and Drug Administration (FDA) for the treatment of invasive
aspergillosis and invasive mucormycosis, available as both oral
and intravenous formulations. Isavuconazonium sulfate is a
water-soluble prodrug, which is rapidly hydrolyzed by esterases
(mainly butylcholinesterase) in plasma into the active moiety
isavuconazole (BAL-4815) and an inactive cleavage product
(BAL-8728). Isavuconazole inhibits cytochrome P450 (CYP)-
dependent enzyme lanosterol 14-ademethylase (CYP51) and
thereby inhibits the synthesis of ergosterol, a key component of
the fungal cell membrane.4 Isavuconazole displayed potent
fungistatic or fungicidal activity in vitro against a broad range of
clinically important yeasts and molds, namely Candida spp.,
Cryptococcus spp., Trichosporon spp., Geotrichum capitatum,
Pichia spp., Rhodotorula spp., Saccharomyces cerevisiae, Aspergillus
spp., and most species known to cause mucormycosis
(Mucorales mucorales). This broad range of antifungal activity renders this drug more clinically appealing compared to other
azoles with narrower indications. Furthermore, isavuconazole
does not require a cyclodextrin vehicle due to its water
solubility, and currently does not require therapeutic drug
monitoring. Moreover, isavuconazole has displayed improved
safety and tolerability compared to voriconazole.
合成
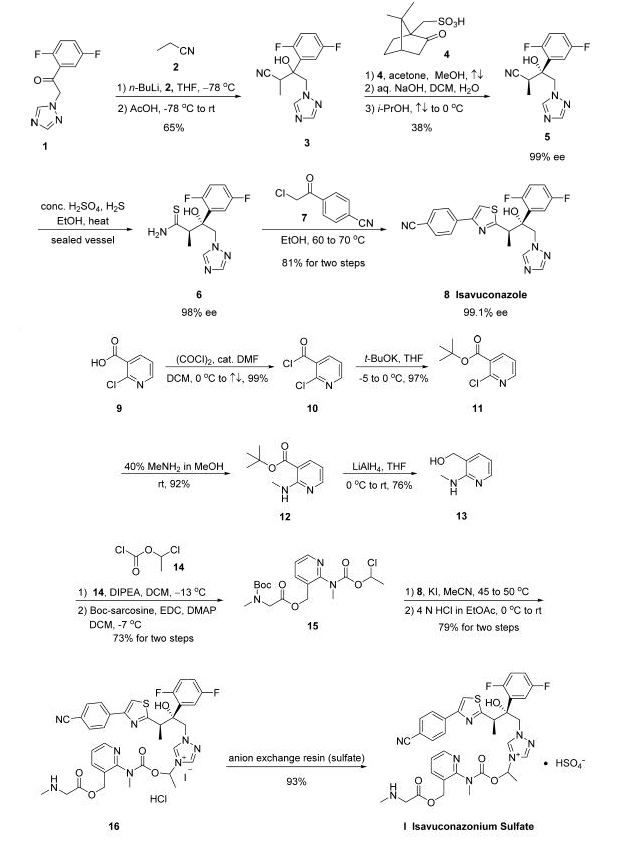
The synthesis of active moiety isavuconazole 8 was started
with commercial 1-(2,5-difluorophenyl)-2-(1H-l,2,4-triazol-lyl)
ethanone (1). Triazole 1 was
treated with n-BuLi followed by exposure to propionitrile (2)
and acidic quench to give racemic alcohol 3 in 65% yield. Next,
resolution of this racemic alcohol was facilitated through the
use of camphor derivative 4 to provide alcohol 5 in 38% yield
and 99% ee. Nitrile 5 was then treated with concentrated
H2SO4 and H2S to furnish thioamide 6, and this was followed
by a cyclization reaction involving 4-(2-chloroacetyl)-
benzonitrile (7) which gave rise to isavuconazole 8 in 81%
yield across the two-step sequence.
The preparation of water-soluble side chain 15
was initiated from commercially available 2-chloronicotinic acid
(9), which was converted to the corresponding tert-butyl ester
11 via acid halide 10 in excellent yield for the two-step
protocol. Subjection of pyridyl chloride 11 to methanolic
methylamine furnished aminopyridine 12 in 92% yield, and this
compound was subsequently reduced with lithium aluminum
hydride to give aminoalcohol 13 in 76% yield. Next, Nacylation
of 13 with 1-chloroethyl chloroformate (14) followed
by treatment with N-Boc-sarcosine under esterification
conditions delivered chloroethyl ester 15 in 73% yield. The
union of the aminopyridyl side chain 15 with thiazoloalcohol 8
was facilitated by reacting the two compounds in the presence
of KI in acetonitrile, and this alkylation was followed by
removal of the Boc group with hydrochloric acid to give rise to
isavuconazonium iodide hydrochloride (16) in 79% yield.
Finally, isavuconazonium sulfate (I) was prepared from 16
using an anion exchange resin in 93% yield to finish the
construction of the API.
アイサブコナゾニウム 上流と下流の製品情報
原材料
準備製品