レゴラフェニブ水和物
レゴラフェニブ水和物 物理性質
- 貯蔵温度 :
- 2-8°C
- 溶解性:
- ≥25.05 mg/mL in DMSO; insoluble in H2O
- 外見 :
- solid
- 色:
- Light yellow to orange
- InChIKey:
- ZOPOQLDXFHBOIH-UHFFFAOYSA-N
- SMILES:
- O(C1C=CC(NC(=O)NC2C=CC(Cl)=C(C(F)(F)F)C=2)=C(F)C=1)C1=CC=NC(C(=O)NC)=C1.O
安全性情報
- リスクと安全性に関する声明
- 危険有害性情報のコード(GHS)
レゴラフェニブ水和物 価格
| メーカー |
製品番号 |
製品説明 |
CAS番号 |
包装 |
価格 |
更新時間 |
購入 |
レゴラフェニブ水和物 化学特性,用途語,生産方法
効能
抗悪性腫瘍薬, 受容体チロシンキナーゼ阻害薬
商品名
スチバーガ (バイエル薬品)
説明
Regorafenib monohydrate (BAY 73-4506) is an orally active and potent multi-targeted receptor tyrosine kinase inhibitor with potent anti-tumour and anti-angiogenic activity. It is approved for the treatment of metastatic colorectal cancer, advanced gastrointestinal mesenchymal stromal tumours and hepatocellular carcinoma.
臨床応用
Regorafenib was approved by the U.S. Food and Drug Administration (FDA) in September 2012 for
the treatment of metastatic colorectal cancer in patients who have previously undergone
fluoropyrimidine-, oxaliplatin-, and irinotecan-based therapies. The FDA expanded the approved use
of the drug to include patients with advanced gastrointestinal stromal tumors (GIST) that cannot be
surgically removed and no longer respond to imatinib and sunitinib, two other drugs approved for
treatment of GIST. Regorafenib, marketed under the trade name Stivarga®, was discovered and
developed by Bayer Pharmaceuticals and marketed jointly with Onyx Pharmaceuticals. The active
metabolites of the drug inhibit multiple targets within a variety of kinase families including those in the RET, VEGF, FGFR, PTK, and Abl pathways.
合成
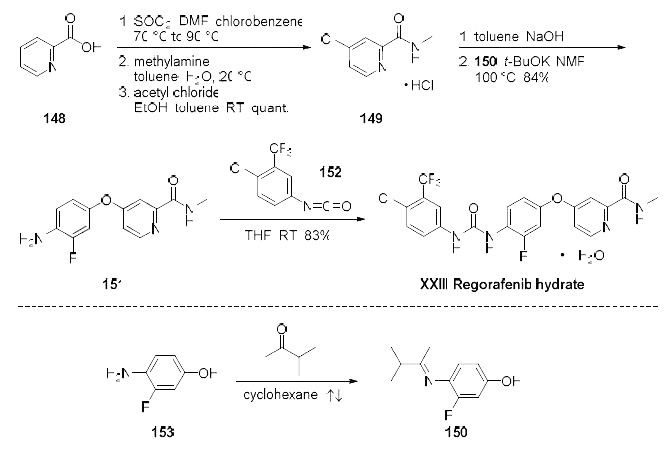
Among several published synthesis, the most likely process scale synthesis will be highlighted
from the two published syntheses, and this is described in the scheme. Commercially available
picolinic acid (148) was heated with thionyl chloride to provide the crude intermediate 4-chloro-2-
pyridyl acid chloride which was subsequently reacted with aqueous methyl amine in toluene to give 4-
chloro-2-methylcarboxamide as its hydrochloride salt 149 in quantitative yield after treatment with
acetyl chloride in toluene and ethanol. The hydrochloride salt was free based with sodium hydroxide
and then immediately reacted with imine 150 (formed upon exposure to 4-amino-3-fluorophenol (153)
in refluxing 3-methyl 2-butanone) in base to provide diaryl ether 151 in 84% yield. Reaction of amine
151 with the commercially available isocyanate 152 ultimately delivered regorafenib hydrate (XXIII) in
83% yield.
レゴラフェニブ水和物 上流と下流の製品情報
原材料
準備製品
レゴラフェニブ水和物 生産企業
Global( 245)Suppliers
レゴラフェニブ水和物 スペクトルデータ(1HNMR)
1019206-88-2(レゴラフェニブ水和物)キーワード:
- 1019206-88-2
- 4-[4-[[[[4-Chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]-3-fluorophenoxy]-N-methyl-2-pyridinecarboxamide hydrate
- Regorafenib monohydrate
- Regorafenib (BAY 73-4506)Monohydrate
- Regorafenib hydrate
- BAY 73-4506 Monohydrate
- Regorafenib monohydrate 4-[4-[[[[4-Chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]-3-fluorophenoxy]-N-methyl-2-pyridinecarboxamide hydrate
- 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methyl-2-pyridinecarboxamide hydrate (1:1)
- 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]-3-fluorophenoxy]-N-methylpyridine-2-carboxamide,hydrate
- Regorafenib monohydrate (BAY 73-4506)
- Regorafenib H2O
- 4-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide hydrate
- Regorafenibhydrate USP/EP/BP
- 4-[4-[[[[4-Chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]-3-fluorophenoxy]-N-methyl-2-pyridinecarboxamide hydrate USP/EP/BP
- 4-[4-[[[[4-Chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]-3-fluorophenoxy]-N-methyl-2-pyridi
- Refined Sunflower Oil
- Regfenib monohydrate
- Regorafenib (200 mg)
- 4-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide hydrate , Regorafenib monohydrate
- Regfini hydrate
- レゴラフェニブ水和物
- レゴラフェニブ水和物 (JAN)