ペンタン 化学特性,用途語,生産方法
外観
無色澄明の液体
定義
ペンタンは,アルカンに属する炭素5個の炭化水素の総称。n-ペンタン、イソペンタン(2-メチルブタン)、ネオペンタン(2,2-ジメチルプロパン)の3種の異性体が知られているが、普通、ペンタンというと直鎖状ペンタン、すなわちn-ペンタンをさす。n-ペンタンは石油や天然ガス中に含まれる。石油エーテルの主成分である。ガソリンに似たにおいをもつ可燃性液体で爆発限界は1.4~8.0%(空気中)。自動発火点は309℃。アルコール、エーテルにも溶ける。濃い蒸気は麻酔性を示す。マウスに対する致死量は12万8200ppmである。抽出溶剤、麻酔剤などに用いられる。イソペンタン、ネオペンタンは非常に気化しやすい液体で、ほとんど無臭である。
定義
本品は、次の化学式で表される炭化水素である。
溶解性
エタノール及びアセトンに極めて溶けやすく、水にほとんど溶けない。
解説
C5H12(72.15).CH3(CH2)3CH3.5個の炭素原子が直鎖状に結合したもので,異性体であるイソペンタン,ネオペンタンと区別して,n-ペンタンとよばれることも多い.また,これらの異性体のすべて,C5H12で表される脂肪族炭化水素の総称としても用いられる.原油および石油系炭化水素分解油中に存在する.実験室的には,tert-ペンチルアルコールと臭化水素酸とで得られる2-ブロモペンタンに,マグネシウムをブチルエーテル中で反応させ,得られた生成物を硫酸で分解して合成する.工業的には,ナフサおよび上記分解油中から分留により分離すると得られる.芳香をもつ引火性の無色の液体.融点-129.72 ℃,沸点36.07 ℃.d425"0.62139.nD25"1.35472.爆発範囲1.4~7.8体積%.炭化水素,油脂,エーテルに可溶,エタノールに微溶,水に不溶.用途はおもに抽出用溶剤に使用されるほかに,低温温度計,麻酔剤として使われる.[CAS 109-66-0]
森北出版「化学辞典(第2版)
用途
分光、ガスクロマト分析、溶剤、化粧品原料 イソペンタンとの混合物、樹脂の発泡剤、金属洗浄剤、接着剤、印刷インキ溶剤
用途
製氷用溶媒、抽出用溶剤
用途
低沸点溶剤、洗剤及び接着剤添加剤。
用途
低沸点溶剤、機器分析の標準。
化粧品の成分用途
減粘剤、噴射剤、溶剤
主な用途/役割
溶剤型接着剤、エマルション系接着剤、エアゾール接着剤に使用される。
使用上の注意
不活性ガス封入
化学的特性
n-Pentane is a flammable liquid. It has applications in industry as an aerosol propellant and as an important component of engine fuel.
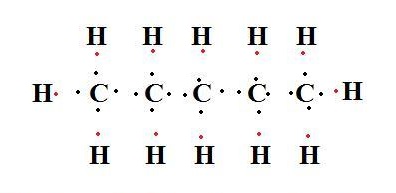
pentane lewis structure
N-propane is a CNS depressant. Studies with dogs have indicated that it induces cardiac sensitization. In high concentrations it causes incoordination and inhibition of the righting refl exes.
N-pentane is being used primarily in Europe in integral-skin flexible foams. Pentane is twice as effective as CFC-11 as a blowing agent.
物理的性質
Clear, colorless, volatile liquid with an odor resembling gasoline. An odor threshold concentration
of 1.4 ppm
v was reported by Nagata and Takeuchi (1990).
使用
n-Pentane occurs in volatile petroleum fractions(gasoline) and as a constituent ofpetroleum ether. It is used as a solvent, in themanufacture of low-temperature thermometers,and as a blowing agent for plastics.
定義
pentane: A straight-chain alkanehydrocarbon, C5H12; r.d. 0.63; m.p.–129.7°C; b.p. 36.1°C. It is obtainedby distillation of petroleum.
調製方法
Pentane is produced by fractional distillation of natural gas
liquids and crude oil. It is also produced by the catalytic
crackdown of naphtha.
一般的な説明
A clear colorless liquid with a petroleum-like odor. Flash point 57°F. Boiling point 97°F. Less dense than water and insoluble in water. Hence floats on water. Vapors are heavier than air.
空気と水の反応
Highly flammable. Insoluble in water.
反応プロフィール
Pentane is incompatible with strong oxidizers. Pentane is also incompatible with strong acids, alkalis, and oxygen. Mixtures with chlorine gas have produced explosions. Pentane will attack some forms of plastics, rubber, and coatings. .
健康ハザード
n-Pentane did not exhibit any marked toxicityin animals. However, inhalation ofits vapors at high concentrations can causenarcosis and irritation of the respiratorypassages. Such effects may be observedwithin the range 5–10% concentration inair. In humans, inhalation of 5000 ppm for10 minutes did not cause respiratory tractirritation or other symptoms (Patty and Yant1929).
There is no report in the literature indicatingany adverse effects from pentaneother than narcosis and irritation. An intravenousLD50 value in mouse is recorded as446 mg/kg (NIOSH 1986).
火災危険
Behavior in Fire: Containers may explode
化学反応性
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
安全性プロファイル
Moderately toxic by inhalation and intravenous routes. Narcotic in high concentration. The liquid can cause blisters on contact. Flammable liquid. Highly dangerous fire hazard when exposed to heat, flame, or oxidizers. Severe explosion hazard when exposed to heat or flame. Shock can shatter metal containers and release contents. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes
職業ばく露
Pentane is used in manufacture of ice,
low-temperature thermometers; in solvent extraction
processes; as a blowing agent in plastics; as a fuel; as a
chemical intermediate (for amylchlorides, e.g.).
環境運命予測
Biological. n-Pentane may biodegrade in two ways. The first is the formation of pentyl
hydroperoxide, which decomposes to 1-pentanol followed by oxidation to pentanoic acid. The
other pathway involves dehydrogenation to 1-pentene, which may react with water giving 1-
pentanol (Dugan, 1972). Microorganisms can oxidize alkanes under aerobic conditions (Singer
and Finnerty, 1984). The most common degradative pathway involves the oxidation of the
terminal methyl group forming 1-pentanol. The alcohol may undergo a series of dehydrogenation
steps forming an aldehyde (valeraldehyde) then a fatty acid (valeric acid). The fatty acid may then
be metabolized by β-oxidation to form the mineralization products, carbon dioxide and water
(Singer and Finnerty, 1984). Mycobacterium smegnatis was capable of degrading pentane to 2-
pentanone (Riser-Roberts, 1992).
Photolytic. When synthetic air containing gaseous nitrous acid and pentane was exposed to
artificial sunlight (λ = 300–450 nm) methyl nitrate, pentyl nitrate, peroxyacetal nitrate, and
peroxypropionyl nitrate formed as products (Cox et al., 1980).
Chemical/Physical. Complete combustion in air yields carbon dioxide and water. Pentane will
not hydrolyze because it does not contain a hydrolyzable functional group.
輸送方法
UN1265 Pentanes Hazard Class: 3; Labels:
3-Flammable liquid.
合成方法
ベンゼンの水素化の副反応により生成する
純化方法
Stir the pentane with successive portions of conc H2SO4 until there is no further coloration during 12hours, then with 0.5N KMnO4 in 3M H2SO4 for 12hours, wash with water and aqueous NaHCO3. Dry it with MgSO4 or Na2SO4, then P2O5 and fractionally distil it through a column packed with glass helices. It is also purified by passage through a column of silica gel, followed by distillation and storage with sodium hydride. An alternative purification is by azeotropic distillation with MeOH, which is subsequently washed out from the distillate (using water), followed by drying and re-distilling. For removal of carbonyl-containing impurities, see n-heptane. Also purify it by fractional freezing (ca 40%) on a copper coil through which cold air is passed, then wash with conc H2SO4 and fractionally distil it. [Beilstein 1 IV 303.]
不和合性
Vapors may form explosive mixture with
air. Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides. Attacks some plastics, rubbers, and
coatings.
廃棄物の処理
Dissolve or mix the
material with a combustible solvent and burn in a chemical
incinerator equipped with an afterburner and scrubber.
All federal, state, and local environmental regulations
must be observed.
ペンタン 上流と下流の製品情報
原材料
準備製品
5-METHOXY-QUINAZOLIN-4-YLAMINE
1-ヘプタノール
2-アミノ-5-ニトロピリミジン
1,4-ジブロモペンタン
2-Bromo-5-hydroxypyridine radical ion(1+)
3-アミノピリダジン塩酸塩
トリス〔3-(トリフルオロメチルヒドロキシメチレン)-D-カンフォラト〕ユウロピウム(III)
セフェピム
2-[2-[2-(2-ブトキシエトキシ)エトキシ]エトキシ]エタノール
ジ-tert-ブチルクロロホスフィン
りん酸7-クロロビシクロ[3.2.0]ヘプタ-2,6-ジエン-6-イルジメチル
1-シクロペンテンカルボン酸
オルノプロスチル
4-BROMO-3-(TRIFLUOROMETHYL)-1-PHENYL-1H-PYRAZOLE
2,2,4,6,7-ペンタメチルジヒドロベンゾフラン-5-スルホニルクロリド
(1R,4R)-1,7,7-トリメチル-3-(トリフルオロアセチル)ビシクロ[2.2.1]ヘプタン-2-オン
tert-ブチルジメチルクロロシラン
1-AMINOCYCLOPROPANECARBONITRILE
ケタミン·塩酸塩
ビス(ピナコラト)ジボロン
4-ベンジルオキシフェニルボロン酸
1-TERT-BUTYL-3-(TRIFLUOROMETHYL)-1H-PYRAZOLE-4-CARBOXYLIC ACID
4,6-ジヒドロキシ-5-メチルピリミジン
2,4,6-トリイソプロピルベンゼンスルホニルクロリド
2-メトキシフェノキシ酢酸
フェキソフェナジン
ジ-tert-ブチルジクロロシラン
イソペンタン
1-ブロモ-4-メトキシブタン
2-AMINOPENT-4-YNENITRILE
シクロブタノール
tert-ブチル N-(4-ホルミルピリジン-3-イル)カルバマート
シクロペンタン
ETHYL 1-TERT-BUTYL-3-(TRIFLUOROMETHYL)-1H-PYRAZOLE-4-CARBOXYLATE
2-(4-ブロモフェニル)-2-メチルプロピオン酸
トリメチルシリル酢酸
4-ブロモ-1-メチル-3-(トリフルオロメチル)-1H-ピラゾール
1-TERT-BUTYL-4-BROMO-3-(TRIFLUOROMETHYL)-1H-PYRAZOLE
α-メチル-L-プロリン
クロロメチル メチル スルフィド