オイゲノール 化学特性,用途語,生産方法
外観
無色〜黄色, 澄明の液体
定義
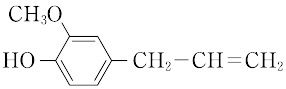
本品は、次の化学式で表されるフェノール誘導体である。
溶解性
水に不溶, エタノール, アセトンに易溶。エタノール及びアセトンに溶けやすく、水にほとんど溶けない。
解説
オイゲノール精油成分の一つで,ちょうじ油,肉けい葉油,けい皮油などに含まれる.
"ちょうじ油様の香気をもち,水に不溶,普通の有機溶媒に可溶.沸点255 ℃.d15"1.072.nD20"1.541~1.542.香水などの香料として用いられる.[CAS 97-53-0]
森北出版「化学辞典(第2版)
用途
オイゲノール用途は芳香剤および香味剤である[IARC 36 (1985)]。
化粧品の成分用途
変性剤、香料
効能
歯髄鎮痛薬, 消毒薬
説明
Sensitization to eugenol mainly occurs in those in
dental professions. Eugenol is contained in the "fragrance
mix".
化学的特性
Eugenol is the main component
of several essential oils; clove leaf oil and cinnamon leaf oilmay contain>90%.Eugenol occurs in small amounts in many other essential oils. It is a colorless to
slightly yellow liquid with a spicy, clove odor.
Catalytic hydrogenation (e.g., in the presence of noble metal catalysts) yields
dihydroeugenol. Isoeugenol is obtained fromeugenol by shifting the double bond.
Esterification and etherification of the hydroxy group of eugenol yield valuable
fragrance and flavor materials (e.g., eugenol acetate and eugenol methyl ether).
天然物の起源
Reported found as a constituent in several volatile oils: clove oil, laurel and cinnamon leaf oil. Smaller amounts
of eugenol are also present in the oil of camphor, Java citronella, California laurel and acacia flowers; remarkable amounts of eugenol
are found in Ocimum sanctum (70%) and Ocimum gratissimum (60%). Eugenol is also found in the oil from violet flowers (21%);
in some plants, eugenol probably occurs as glucoside. Reported found in apricot, citrus oils, raspberry, strawberry, tomato, anise,
cinnamon (leaf, bark and roots), clove bud and stem, nutmeg, mace, pepper, smoked fish, beer, whiskey, grape wines, cocoa, mango,
tarragon, laurel, myrtle leaf, and pimento berry and leaf.
使用
Eugenol is a dental compound which shows cytotoxicity to human oral squamous cell carcinoma and oral cells. When glucosylated, this compound exhibits anti-inflammatory activity.
製造方法
Since sufficient eugenol can be isolated from cheap essential oils,
synthesis is not industrially important. Eugenol is still preferentially isolated from
clove leaf and cinnamon leaf oil (e.g., by extraction with sodium hydroxide solution).
Nonphenolic materials are then removed by steam distillation. After the
alkaline solution is acidified at low temperature, pure eugenol is obtained by distillation.
定義
ChEBI: A phenylpropanoid formally derived from guaiacol with an allyl chain substituted para to the hydroxy group.
一般的な説明
Clear colorless pale yellow or amber-colored liquid. Odor of cloves. Spicy pungent taste.
空気と水の反応
Darkens and thickens on exposure to air. Also darkens with age. Eugenol may decompose on exposure to light. Insoluble in water.
反応プロフィール
Eugenol is incompatible with strong oxidizers. This includes ferric chloride and potassium permanganate. Eugenol reacts with strong alkalis. Eugenol is incompatible with iron and zinc.
危険性
Questionable carcinogen.
火災危険
Eugenol is combustible.
接触アレルゲン
Eugenol is a fragrance allergen obtained from many
natural sources. Occupational sensitization to eugenol
may occur in dental profession workers. Eugenol is
contained in “fragrance mix” and has to be listed by
name in cosmetics within the EU.
臨床応用
4-Allyl-2-methoxyphenol is obtained primarily from cloveoil. It is a pale-yellow liquid with a strong aroma of clovesand a pungent taste. Eugenol is only slightly soluble in waterbut is miscible with alcohol and other organic solvents.Eugenol possesses both local anesthetic and antiseptic activityand can be directly applied on a piece of cotton to relievetoothaches. Eugenol is also used in mouthwashes because ofits antiseptic property and pleasant taste. The phenol coefficientof eugenol is 14.4.
抗がん研究
This compound was tested on a model of skin tumor induced by DMBA croton oilin Swiss mice. The eugenol affects the cellular proliferation by increasing apoptosiscellular death. There is evidence for a downregulation of c-myc, H-ras, and Bcl-2expression and an upregulation of p53, Bax, and active caspase-3 (Grondona et al.2014).
安全性プロファイル
Moderately toxic by
ingestion, intraperitoneal, and subcutaneous
routes. Human mutation data reported. A
human skin irritant. Questionable
carcinogen with experimental carcinogenic
and tumorigenic data. Combustible liquid.
When heated to decomposition it emits
acrid smoke and irritating fumes. See also
ALLYL COMPOUNDS.
代謝
No absorption of eugenol occurred within 2hr of application to the intact shaved skin of mice (Meyer & Meyer, 1959). Following ip injec tion of [
14C]eugenol into rats, radioactivity was dis tributed in various organs and the presence of
14CO
2 in the expired air indicated the demethylation of eugenol (Weinberg, Rabinowitz, Zanger & Gennaro, 1972). Over 70% of an oral dose of eugenol was excreted in the urine of rabbits (Schr?der & Vollmer, 1932).
純化方法
Fractional distillation of eugenol gives a pale yellow liquid which darkens and thickens on exposure to air. It should be stored under N2 at -20o. [Waterman & Priedster Recl Trav Chim Pays-Bas 48 1272 1929, Beilstein 6 H 961, 6 IV 6337.]
オイゲノール 上流と下流の製品情報
原材料
準備製品