N-ISOBUTYL-O-BENZOYLHYDROXYLAMINE 化学特性,用途語,生産方法
製造方法
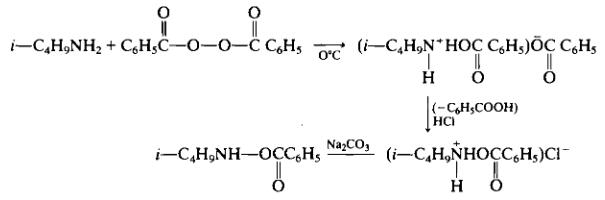
Following the method reported by Zinner [78c] to 29.2 g (0.4 mole) of isobutylamine dissolved in 40 ml benzene (suggest toluene instead) is added a solution of 48.4 g (0.2 mole) of dibenzoyl peroxide in 200 ml benzene (suggest toluene instead), with stirring and cooling to 0°C. The yellow-colored reaction was first heated for 30 min at 30°C and then 30 min at 50°C. After cooling to 0° N-isobutylammonium benzoate separates as a white precipitate. The filtrate is washed with water and dried over magnesium sulfate. The mixture is filtered, and then anhydrous hydrogen chloride is bubbled through the solution to give 12.1 g (26% yield) of a white precipitate of TV-isobutyl-O-benzoylhydroxylamine hydrochloride, m.p. 120-128°C. The infrared spectrum in Nujol indicated an absorbtion band at 1760 cm
-1.
Treatment of the latter product with IN aqueous sodium carbonate and then extraction with ether, drying over sodium sulfate and evaporation gave N-isobutyl-O-benzoylhydroxylamine. Precipitation of the latter by Kugelrohr distillation gave product b.p. 58-65°C (0.04-0.03 mm Hg). The IR (CCU) indicated 3235, 1725, 1267, 1088, 1067, 1027 cm
-1; and the NMR (CCI4) indicated δ, 0.99(rf, J = 6.5Hz. 6H), 1.83 (m, J = 6.5Hz, 1H), 2.87 (d, J = 6.5 Hz, 2H), 7.47 and 7.98 (aromatic m and NH). K. Reactions of Grignard Reagents A variety of nitrogen-containing compounds have been treated with Grignard reagents in an effort to produce substituted hydroxylamines. Many of these reactions have not been explored extensively and no reasoned judgement as to general applicability can be made. These methods were presented in outline form in Schemes 2 and 3. L. Reaction of Chloramine with Alcoholates The reaction of chloramine and an alcoholate according to Eq. (46) appears attractive since it involves the direct formation of the oxygen-to-nitrogen bond in an O-alkylated hydroxylamine.
N-ISOBUTYL-O-BENZOYLHYDROXYLAMINE 上流と下流の製品情報
原材料
準備製品