tert-ブチルジフェニルクロロシラン 化学特性,用途語,生産方法
外観
僅かにうすい黄色~暗赤色、液体
用途
アルコールの保護シリル化剤。
使用上の注意
不活性ガス封入。
化学的特性
Tert-Butylchlorodiphenylsilane is a colorless to pale brown oily liquid with pungent odor, may be used as silylating agent for derivatization of alcohols, ketones, carboxylic acids, amines, amides and mercaptanes selectively into functional groups in different sterical environments.
物理的性質
colorless liquid, bp 93–95°C/0.015 mmHg;
n20
D 1.5680; d 1.057 g cm?3.
使用
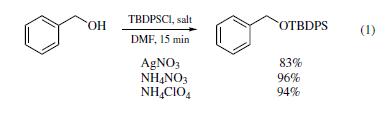
Several newmethods have been developed
for using the reagent to protect primary and secondary alcohols
as their TBDPS ethers. In the presence of ammonium nitrate or
ammonium perchlorate, reaction between TBDPS-Cl and a primary
alcohol, such as benzyl alcohol, in DMF provided excellent
yields of the corresponding silyl ethers in just 15 min (eq 1).19
When silver nitrate was used as promoter, the reactions gave
inferior yields under otherwise identical conditions.
When TBDPS-Cl is used to react with hemiacetals, it converts
hemiacetals into ring-opened silyl ether carbonyl compounds, instead
of mixed acetals.Presumably, the sizable TBDPS group
presents too much steric hindrance for the formation of the corresponding
mixed silyl acetals.
製造方法
a dry 1 L, three-necked round bottomed
flask is equipped with a magnetic stirring bar, a 500mL equalizing
dropping funnel fitted with a rubber septum, a reflux condenser,
and nitrogen inlet tube. The flask is flushed with nitrogen,
then charged with 127 g (0.5 mol) of diphenyldichlorosilane
in 300mL of redistilled pentane. A solution of tbutyllithium
in pentane (500 mL, 0.55 mol), is transferred under
nitrogen pressure to the dropping funnel using a stainless steel,
double-tip transfer needle. This solution is slowly added to the
contents of the flask and when the addition is complete, the
mixture is refluxed 30 h under nitrogen with stirring. The suspension
is allowed to cool to rt, the precipitated lithium chloride
is rapidly filtered through a pad of Celite, and the latter
is washed with 200mL of pentane. The solvent is removed by
evaporation, and the colorless residue is distilled through a short
(10 cm), Vigreux column, to give 125–132 g of the colorless title
compound.
純化方法
Purify it by repeated fractional distillaton. It is soluble in DMF and pentane [Hanessian & Lavalee Can J Chem 53 2975 1975, Robl et al. J Med Chem 34 2804 1991]. [Beilstein 4 IV 4076 for tert-butylchlorodimethylsilane.]
tert-ブチルジフェニルクロロシラン 上流と下流の製品情報
原材料
準備製品
フルバスタチンナトリウム水和物
3-AMINO-2,2-DIMETHYL-4-OXO-AZETIDINE-1-SULFONIC ACID
2-(t-Butyldiphenylsilanyloxy)Ethanol
(S)-4-BOC-MORPHOLINE-3-CARBOXYLIC ACID
4-ペンチルシクロヘキサノン
tert-ブチルジフェニル(2-ブロモエトキシ)シラン
(S)-2-(TERT-BUTOXYCARBONYLAMINO)-3-(TERT-BUTYLDIPHENYLSILYLOXY)PROPANOIC ACID
(ブト-3-エン-1-イルオキシ)(TERT-ブチル)ジフェニルシラン
Benzene, 1,1'-[(1,1-dimethylethyl)(2-propen-1-yloxy)silylene]bis-
2-(2-((tert-butyldiphenylsilyl)oxy)ethoxy)ethanol(WXPC0004)
6-O-(TERT-ブチルジフェニルシリル)-D-グルカール
METHYL-(6R)-((T-BUTYL) DIPHENYLSILYLOXY)-(2E,4E,8Z)-TETRADECATRIENOATE
2,6-DI([1-(TERT-BUTYL)-1,1-DIPHENYLSILYL]OXY)-9,10-DIHYDROANTHRACENE-9,10-DIONE
4-(tert-ブチルジフェニルシロキシ)-1-ブチン