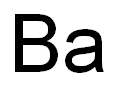Barium suppliers
Barium
- CAS:
- 7440-39-3
- MF:
- Ba
- MW:
- 137.33
Suppliers by country/region
Company Type
Properties
- Melting point:
- 725 °C(lit.)
- Boiling point:
- 1640 °C(lit.)
- Density
- 3.6 g/mL at 25 °C(lit.)
- storage temp.
- water-free area
- solubility
- reacts with H2O; slightly soluble in ethanol
- form
- rod
- Specific Gravity
- 3.51
- color
- Silver-gray
- PH
- 0.5 (20°C in H2O)
- Flame Color
- Light apple green
- Resistivity
- 50.0 μΩ-cm, 20°C
- Water Solubility
- soluble with H2 evolution in cold H2O and hot H2O; slightly soluble alcohol; insoluble benzene [CRC10]
- Sensitive
- air sensitive, moisture sensitive
- Merck
- 13,967
- Exposure limits
- TLV-TWA 0.5 mg/m3 (for soluble compounds) (ACGIH and MSHA); IDLH (for soluble compounds) 250 mg/m3 (NIOSH). .
- Stability:
- Stability Reacts vigorously or violently with acids, water, tetrachloromethane, small halogenated hydrocarbons. Should be stored under an inert material such as petroleum ether to exclude air. Flammable.
- CAS DataBase Reference
- 7440-39-3(CAS DataBase Reference)
- EPA Substance Registry System
- Barium (7440-39-3)
Safety Information
- Symbol(GHS)



GHS02,GHS05,GHS06
- Signal word
- Danger
- Hazard statements
- H228-H260-H301-H314
- Precautionary statements
- P210-P231+P232-P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338
- Hazard Codes
- C,Xi,F
- Risk Statements
- 25-26-34-36/37/38-14/15-11
- Safety Statements
- 23-26-36-36/37/39-45-43-36/37-16
- RIDADR
- UN 3264 8/PG 3
- OEB
- C
- OEL
- TWA: 0.5 mg/m3
- WGK Germany
- 3
- RTECS
- CQ8370000
- TSCA
- Yes
- HS Code
- 2805 19 10
- HazardClass
- 8
- PackingGroup
- III
- Hazardous Substances Data
- 7440-39-3(Hazardous Substances Data)
- Toxicity
- An element; the heaviest of the stable alkaline earths. Barium sulfate is used as a diagnostic aid in radiology due to its radio-opaqueness and, because of its insolubility and lack of absorption, it is safe barring iatrogenic episodes. Poisoning usually results from deliberate or accidental ingestion of soluble barium compounds. The Ba2+ ion is a muscle poison due to the blocking of the K1 channels of the Na+/K+ pump in cell membranes. Because cases of barium poisoning are accompanied by severe hypokalemia, potassium infusion is an effective antidote. The toxicity of barium compounds depends on their solubility, with the free ion being readily absorbed from gastrointestinal tract or lung, whereas the sulfate is essentially unabsorbed. Thus, administration of soluble sulfates immediately after ingestion is another effective antidote.
- IDLA
- 50 mg Ba/m3
Use
Barium is found in various alloys, paints, soap, paper, photographic chemicals, explosives, and rubber, and is used in the manufacture of ceramics and glass. Some of its compounds are used as mordants in fabric dyeing and in the preparation of phosphors. One major use is in slurry of ground barite (ZnS + BaSO4) for gas and oil drilling. Barium fluorosilicate has been used as an insecticide, and some barium compounds are used as rodenticides. Medicinally, barium sulfate, being very sparingly soluble, is used as a radiopaque contrast material for X-ray diagnostic purposes and other medical imaging uses.
134 supplier list of "Barium"
- Product Name:barium
- Company Type: Reagent
- Country/Region: CHINA
- Main Products: Gamma-butyrolactone
- Product Name:Barium
- Products Intro:Purity: 99.9% metals basis | Package: $222.9/5g;$484.9/100g;$887.9/25g;Bulk package | CustNote: 99.9% metals basis
- Company Type: Trader
- Country/Region: UNITED STATES
- Main Products: Chemicals and BioChemicals,Materials Science,ligands acting on targets,Proteins,Antibodies
You can find Barium suppliers,
manufacturers, and distributors from countries such as United States, CHINA and the United Kingdom here.
Browse to the tetrahydrofuran product information displayed by the supplier,
as well as the supplier's contact and capability information.