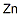ZINC suppliers
ZINC
- CAS:
- 7440-66-6
- MF:
- Zn
- MW:
- 65.39
Properties
- Melting point:
- 420 °C(lit.)
- Boiling point:
- 907 °C(lit.)
- Density
- 7.14 g/mL at 25 °C
- bulk density
- 1800-2700kg/m3
- vapor pressure
- 1 mm Hg ( 487 °C)
- Flash point:
- 1 °F
- storage temp.
- 2-8°C
- solubility
- H2O: soluble
- form
- wire
- Specific Gravity
- 7.14
- color
- Silvery-gray
- Flame Color
- Colorless to blue-green
- Odor
- at 100.00?%. odorless
- Resistivity
- 5.8 μΩ-cm, 20°C
- Water Solubility
- Soluble in water.
- Sensitive
- Air & Moisture Sensitive
- Merck
- 14,10132
- Exposure limits
- ACGIH: TWA 2 ppm; STEL 4 ppm
OSHA: TWA 2 ppm(5 mg/m3)
NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3)
- Stability:
- Stable. Incompatible with amines, cadmium, sulfur, chlorinated solvents, strong acids, strong bases. Air and moisture sensitive. Zinc powder is very flammable.
- InChIKey
- HCHKCACWOHOZIP-UHFFFAOYSA-N
- CAS DataBase Reference
- 7440-66-6(CAS DataBase Reference)
- NIST Chemistry Reference
- Zinc(7440-66-6)
- EPA Substance Registry System
- Zinc (7440-66-6)
Safety Information
- Symbol(GHS)




GHS02,GHS07,GHS08,GHS09
- Signal word
- Danger
- Hazard statements
- H225-H302-H319-H335-H336-H351-H411
- Precautionary statements
- P202-P210-P273-P301+P312-P305+P351+P338-P308+P313
- Hazard Codes
- N,F,Xi,Xn
- Risk Statements
- 52/53-50/53-17-15-36/37/38-51/53-36/37-22-19-40-11
- Safety Statements
- 26-61-60-46-43-36-36/37-16
- RIDADR
- UN 3264 8/PG 3
- WGK Germany
- 3
- RTECS
- ZH1400000
- F
- 3
- Autoignition Temperature
- 460 °C
- TSCA
- Yes
- HS Code
- 7904 00 00
- HazardClass
- 8
- PackingGroup
- III
- Hazardous Substances Data
- 7440-66-6(Hazardous Substances Data)
- Toxicity
- Zinc is an essential nutrient and is not regarded as toxic. However, the metal fumes, its oxide fumes, and chloride fumes can produce adverse inhalation effects. (See Zinc Oxide and Zinc Chloride, Toxicity) Ingestion of soluble salts can cause nausea.
Use
zinc is described as an oligo element, trace element, or micro nutrient. Zinc is believed to accelerate wound healing. It is also considered an anti-oxidant, offering protection against uV radiation. It appears to favor the sulfur uptake in sulfurated amino acids and facilitates the incorporation of cysteine, an amino acid, into the skin. It also has a synergistic effect with vitamins A and e. Zinc is a component of more than 70 metal enzymes. It promotes collagen synthesis in the dermis and keratinization of the corneum layer. Zinc is useful for acne treatments because it lowers sebaceous secretion, and is also used in the treatment of psoriasis.
According to relevant laws, regulations, policies and the provisions of this website, this website does not display the product information!
The source is "易制爆、炸药、化学武器等". If the information is inaccurate, please contact the platform to modify it.
contact information:info@chemicalbook.com 400-158-660