THIONALIDE
|
- $179 - $5375.58
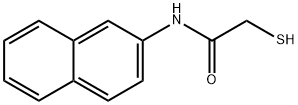
- Product name: THIONALIDE
- CAS: 93-42-5
- MF: C12H11NOS
- MW: 217.29
- EINECS:202-245-0
- MDL Number:MFCD00059137
- Synonyms:ALPHA-MERCAPTO-N,2-NAPHTHYLACETAMIDE;THIONALIDE;2-Mercapto-N-(2-naphtyl)acetamide;N-(2-Naphthalenyl)-2-mercaptoacetamide;Thiolglycolic-β-aminonaphthalide;N-naphthalen-2-yl-2-sulfanylacetamide;N-naphthalen-2-yl-2-sulfanyl-ethanamide;Thionalide [for Cu Determination]
3 prices
Selected condition:
Brand
- American Custom Chemicals Corporation
- Sigma-Aldrich
Package
- 50mg
- 100MG
- 100G
- ManufacturerAmerican Custom Chemicals Corporation
- Product numberCHM0165409
- Product descriptionTHIONALIDE 98.00%
- Packaging100MG
- Price$965.58
- Updated2021-12-16
- Buy
- ManufacturerAmerican Custom Chemicals Corporation
- Product numberCHM0165409
- Product descriptionTHIONALIDE 98.00%
- Packaging100G
- Price$5375.58
- Updated2021-12-16
- Buy
- ManufacturerSigma-Aldrich
- Product numberS860522
- Product description2-MERCAPTO-N-(2-NAPHTHYL)ACETAMIDE Aldrich
- Packaging50mg
- Price$179
- Updated2024-03-01
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| American Custom Chemicals Corporation | CHM0165409 | THIONALIDE 98.00% | 100MG | $965.58 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | CHM0165409 | THIONALIDE 98.00% | 100G | $5375.58 | 2021-12-16 | Buy |
| Sigma-Aldrich | S860522 | 2-MERCAPTO-N-(2-NAPHTHYL)ACETAMIDE Aldrich | 50mg | $179 | 2024-03-01 | Buy |
Properties
Melting point :111-112°
Density :1.1799 (rough estimate)
refractive index :1.6000 (estimate)
solubility :Sparingly soluble in water, readily soluble in organic solvents. The solubility in water is considerably increased by addition of relatively small amounts of ethanol or acetic acid.
form :White or pale yellow needles.
Density :1.1799 (rough estimate)
refractive index :1.6000 (estimate)
solubility :Sparingly soluble in water, readily soluble in organic solvents. The solubility in water is considerably increased by addition of relatively small amounts of ethanol or acetic acid.
form :White or pale yellow needles.
Safety Information
| Symbol(GHS): |
  
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Danger | |||||||||||||||||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
Thionalide reacts with a variety of metal ions to give chelate complexes which are sparingly soluble in water. The difference in stability of complexes formed by the reagent with various metal ions in some cases assures a certain analytical selectivity.1. Copper(II), silver(I), mercury(II), bismuth(III), arsenic(III), tin(IV), gold(I), platinum(IV) and palladium(II) give precipitates with the reagent in acidic media (mineral acids).
2. The copper(II), mercury(II), cadmium(II), thallium(I) and gold(I) complexes precipitate from alkaline solutions containing tartrate.
3. Thallium(I), antimony(III) and bismuth(III) complexes are precipitated by the reagent from solutions containing cyanide and tartrate.
4. The thallium(I) complex also precipitates from alkaline (sodium hydroxide) solution containing cyanide and tartrate.
The above classification shows that the reaction can be made specific for thallium by appropriate choice of the pH of the solution and application of suitable masking agents. Similarly, the selectivity of the precipitation reaction can be increased for other ions too, by application of other masking agents. For instance, tin(IV) can be masked selectively in the presence of arsenic(III) and antimony(III) with phosphoric acid, while these latter two ions can be precipitated with thionalide.
The analytical application of thionalide is stimulated by the fact that its metal complexes are of stoichiometric composition, and can be dried at 105-110°C and weighed directly.
The reagent may also be utilized in volumetric analytical procedures. The thionalide content, which is equivalent to the metal to be determined, can be titrated directly iodometrically, according to the equation: 2C10H7-NH-CO-CH2-SH+I2=C10H7-NH-CO-CH2-S-S-CH2-CO-NH-C10H7+2HI.
More related product prices
Related product price
- THIONALIDE
$179-5375.58 - 2-MERCAPTOACETANILIDE
$199-1328.25





