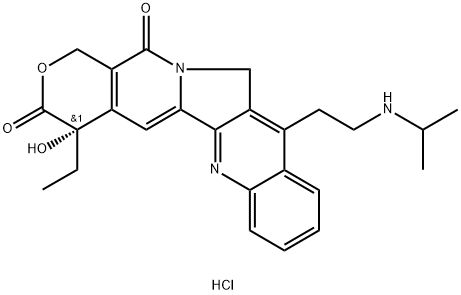
ベロテカン塩酸塩
|
- ¥51300 - ¥394800
- 化学名: ベロテカン塩酸塩
- 英語名: Camtobell hydrochloride
- 別名:ベロテカン塩酸塩
- CAS番号: 213819-48-8
- 分子式: C25H28ClN3O4
- 分子量: 469.97
- EINECS:
- MDL Number:MFCD07772313
2物価
選択条件:
ブランド
- 富士フイルム和光純薬株式会社(wako)
パッケージ
- 10mg
- 100mg
- 生産者富士フイルム和光純薬株式会社(wako)
- 製品番号W01TRCB131300
- 製品説明ベロテカン塩酸塩
- 英語製品説明Belotecan Hydrochloride
- 包装単位10mg
- 価格¥51300
- 更新しました2024-03-01
- 購入
- 生産者富士フイルム和光純薬株式会社(wako)
- 製品番号W01TRCB131300
- 製品説明ベロテカン塩酸塩
- 英語製品説明Belotecan Hydrochloride
- 包装単位100mg
- 価格¥394800
- 更新しました2024-03-01
- 購入
| 生産者 | 製品番号 | 製品説明 | 包装単位 | 価格 | 更新時間 | 購入 |
|---|---|---|---|---|---|---|
| 富士フイルム和光純薬株式会社(wako) | W01TRCB131300 | ベロテカン塩酸塩 Belotecan Hydrochloride |
10mg | ¥51300 | 2024-03-01 | 購入 |
| 富士フイルム和光純薬株式会社(wako) | W01TRCB131300 | ベロテカン塩酸塩 Belotecan Hydrochloride |
100mg | ¥394800 | 2024-03-01 | 購入 |
プロパティ
融点 :>218°C (dec.)
貯蔵温度 :under inert gas (nitrogen or Argon) at 2-8°C
溶解性 :DMSO (Slightly), Methanol (Slightly, heated), Water (Slightly)
外見 :Solid
色 :Pale Beige to Light Brown
貯蔵温度 :under inert gas (nitrogen or Argon) at 2-8°C
溶解性 :DMSO (Slightly), Methanol (Slightly, heated), Water (Slightly)
外見 :Solid
色 :Pale Beige to Light Brown
安全情報
| 絵表示(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注意喚起語: | Warning | ||||||||||||||
| 危険有害性情報: |
|
||||||||||||||
| 注意書き: |
|
説明
Camtobell hydrochloride, a DNA topoisomerase I inhibitor, is an analog of camptothecin. It was launched in the Republic of Korea as an injectable formulation for the treatment of ovarian and small cell lung cancer. Although camptothecin exhibits potent antineoplastic activity in vitro, its clinical application is hampered by severe toxicity and poor water solubility. Several synthetic and semi-synthetic analogs of camptothecin with improved solubility and lower toxicity have been developed over the past two decades. Two drugs from this class, topotecan and irinotecan, have been launched in previous years and belotecan is the newest member to reach the market. Camtobell hydrochloride is prepared by a two-step semi-synthesis starting from camptothecin, first by converting to 7-methylcamptothecin via a free-radical methylation reaction using a combination of acetic acid, tert-butylhydroperoxide, ferrous sulfate and sulfuric acid, and subsequently, in the second step, a Mannich reaction with isopropylamine hydrochloride and dimethylsulfoxide. A total synthesis of belotecan in seventeen steps starting from ethyl acetopyruvate is also reported. Belotecan inhibits topoisomerase I with approximately equal potency as camptothecin and about 3-fold higher potency than topotecan, with respective IC50 values of 0.119, 0.123 and 0.33 μg/mL. Its cytotoxic activity is comparable to that of camptothecin, with IC50 values ranging from 2 ng/mL to 2 μg/mL against 26 different human cancer cell lines. In studies using human tumor xenografts in nude mice, 80-100 mg/ kg of belotecan dosed every four days for four doses produced 67 to 94% tumor regression rates against HT-29, WIDR and CX-1 colon, LX-1 lung, MX-1 breast and SKOV-3 ovarian carcinomas. Pharmacokinetic studies of camtobell hydrochloride in rats at intravenous doses of 2.6–8.9 mg/kg demonstrated that both Cmax and AUC increased in a dose-dependent manner. Total clearances, volumes of distribution and mean residence times did not change significantly with increasing doses. The elimination half-life ranged between 9.2 to 11.2 hours. In a Phase I study of camtobell hydrochloride, the fraction of renal clearance was found to be 33.1 to 50.3%, and the protein-binding fraction was 53 to 87%. Approximately 9.5% of the administered dose was excreted via the hepatobiliary system. In clinical studies involving 20 patients with recurrent or refractory ovarian cancer, intravenous administration of 0.5 mg/m2/day of belotecan for 5 days every 3 weeks over a median of six dosing cycles resulted in an overall response rate of 45%. All patients had grade 3 or 4 neutropenia as the most significant adverse event.関連商品価格
- シアノ酢酸 エチル
¥1300-12540 - ジメチルアミン塩酸塩
¥1200-46000 - ピルビン酸エチル
¥2000-541000




