エンフビルチド (T-20)
|
- ¥23900 - ¥91400
- 化学名: エンフビルチド (T-20)
- 英語名: Enfuvirtide
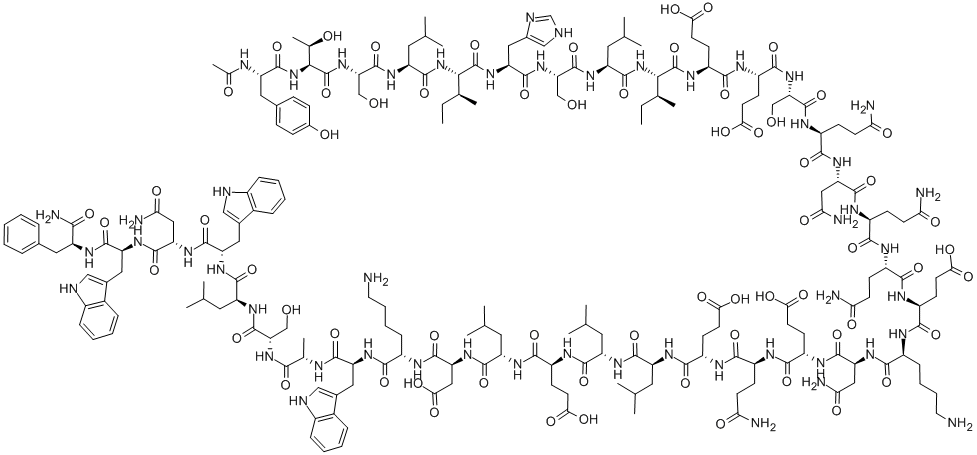
- 別名:エンフビルチド (T-20);N-アセチル-L-Tyr-L-Thr-L-Ser-L-Leu-Ile-L-His-L-Ser-L-Leu-Ile-L-αGlu-L-αGlu-L-Ser-L-Gln-L-Asn-L-Gln-L-Gln-L-αGlu-L-Lys-L-Asn-L-αGlu-L-Gln-L-αGlu-L-Leu-L-Leu-L-αGlu-L-Leu-L-Asp-L-Lys-L-Trp-L-Ala-L-Ser-L-Leu-L-Trp-L-Asn-L-Trp-L-Phe-NH2;フゼオン;エンフビルチド
- CAS番号: 159519-65-0
- 分子式: C204H301N51O64
- 分子量: 4491.92
- EINECS:641-385-0
- MDL Number:MFCD08062320
4物価
選択条件:
ブランド
- 富士フイルム和光純薬株式会社(wako)
- Sigma-Aldrich Japan
パッケージ
- 1mg
- 2mg
- 5mg
- 生産者富士フイルム和光純薬株式会社(wako)
- 製品番号W01LKTE5220
- 製品説明エンフビルチド (T-20)
- 英語製品説明Enfuvirtide (T-20)
- 包装単位5mg
- 価格¥91400
- 更新しました2024-03-01
- 購入
- 生産者富士フイルム和光純薬株式会社(wako)
- 製品番号W01LKTE5220
- 製品説明エンフビルチド (T-20)
- 英語製品説明Enfuvirtide (T-20)
- 包装単位2mg
- 価格¥50800
- 更新しました2024-03-01
- 購入
- 生産者富士フイルム和光純薬株式会社(wako)
- 製品番号W01LKTE5220
- 製品説明エンフビルチド (T-20)
- 英語製品説明Enfuvirtide (T-20)
- 包装単位1mg
- 価格¥30500
- 更新しました2024-03-01
- 購入
- 生産者Sigma-Aldrich Japan
- 製品番号SML0934
- 製品説明 ≥95% (HPLC)
- 英語製品説明Enfuvirtide acetate salt ≥95% (HPLC)
- 包装単位5mg
- 価格¥23900
- 更新しました2024-03-01
- 購入
| 生産者 | 製品番号 | 製品説明 | 包装単位 | 価格 | 更新時間 | 購入 |
|---|---|---|---|---|---|---|
| 富士フイルム和光純薬株式会社(wako) | W01LKTE5220 | エンフビルチド (T-20) Enfuvirtide (T-20) |
5mg | ¥91400 | 2024-03-01 | 購入 |
| 富士フイルム和光純薬株式会社(wako) | W01LKTE5220 | エンフビルチド (T-20) Enfuvirtide (T-20) |
2mg | ¥50800 | 2024-03-01 | 購入 |
| 富士フイルム和光純薬株式会社(wako) | W01LKTE5220 | エンフビルチド (T-20) Enfuvirtide (T-20) |
1mg | ¥30500 | 2024-03-01 | 購入 |
| Sigma-Aldrich Japan | SML0934 | ≥95% (HPLC) Enfuvirtide acetate salt ≥95% (HPLC) |
5mg | ¥23900 | 2024-03-01 | 購入 |
プロパティ
貯蔵温度 :-20°C
溶解性 :DMF: 5 mg/ml; DMSO: 5 mg/ml; PBS (pH 7.2): 2 mg/ml
外見 :powder
色 :white to off-white
シーケンス :Ac-Tyr-Thr-Ser-Leu-Ile-His-Ser-Leu-Ile-Glu-Glu-Ser-Gln-Asn-Gln-Gln-Glu-Lys
溶解性 :DMF: 5 mg/ml; DMSO: 5 mg/ml; PBS (pH 7.2): 2 mg/ml
外見 :powder
色 :white to off-white
シーケンス :Ac-Tyr-Thr-Ser-Leu-Ile-His-Ser-Leu-Ile-Glu-Glu-Ser-Gln-Asn-Gln-Gln-Glu-Lys
安全情報
| 絵表示(GHS): |

|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注意喚起語: | Warning | ||||||||||||||||||||||||||||
| 危険有害性情報: |
|
||||||||||||||||||||||||||||
| 注意書き: |
|
説明
Enfuvirtide is the first of a new class of HIV therapeutics that interferes with the entry of HIV-1 by inhibiting fusion of viral and cellular membranes. It binds to the first heptad-repeat (HR1) in ghe gp41 subunit of the viral envelope glycoprotein and prevents the conformational changes required for the fusion with cell membranes, thus representing a new mechanism. It is a potent inhibitor of this binding with an IC50 in laboratory and primary isolates (HIV-1 clades A–G) ranging from 4 to 280 nM (18–1260 ng/mL), but has virtually no activity against HIV-2. Due to its unique mechanism, it does not show cross-resistance to NRTI, NNRTI and PIs. It is prepared by chemical synthesis, using a combination of solution and solid phase steps. The solid phase steps utilize fmoc protection and the acid sensitive 2- chlorotrityl chloride resin to produce peptides of lengths ranging from nine to sixteen amino acids. These amino acids are subsequently coupled to form the full-length peptide followed by side-chain deprotection and column chromatography. In the HuPBMC-SCID mouse model of HIV-1 infection enfuvirtide showed a dosedependent decrease in viral load. At doses of 200 mg/kg/day, it decreased the RNA levels to 8.2 copies/1 million cells compared to 17 million copies/1 million cells in the saline-treated group. In a clinical study involving 78 heavily pretreated patients (plasma HIV RNA>5000 copies/mL), enfuvirtide dosing of 12.5–200 mg/day by b.i.d. subcutaneous injection resulted in a dose-dependent decrease in viral load (plasma HIV RNA 0.3–1.6 log 10 copies/mL). In a combination drug study where all patients received an optimized retroviral regimen with or without enfuvirtide, it was found that, at week 24, the enfuvirtide group had a 4.5 fold-relative drop in HIV RNA copies/mL relative to the standard treatment group. Subjects received 3–5 antiretroviral agents with or without enfuvirtide. In clinical trials, HIV-1 isolates from 185 patients using enfuvirtide in a cocktail with other antiretroviral agents showed 4 to 422-fold decrease in sensitivity to this drug. These more resistant types had changes in the gp41 amino acids 36–45. Enfuvirtide is dosed twice per day (90 mg) by subcutaneous injection and has an elimination half-life of about 3.8 h. It is highly plasma protein bound (92%) and has a Vdss is 5.5 L. Local injection site irritation (98% had at least one reaction), diarrhea (26.8%), nausea (20.1%), and fatigue (16.1%) were the most common adverse events.関連商品価格
- オクトレオチド·酢酸塩
¥21500-407100 - アバレリックス
¥128700-128700 - 副腎皮質刺激ホルモン放出因子
¥12000-378000
供給者とメーカー
Hebei Yanxi Chemical Co., Ltd.
Henan Bao Enluo International TradeCo.,LTD
Zibo Hangyu Biotechnology Development Co., Ltd
Nantong Guangyuan Chemicl Co,Ltd
Alpha Biopharmaceuticals Co., Ltd
Henan Tianfu Chemical Co.,Ltd.
Rixing Chemical CO.,LTD
career henan chemical co
Shenzhen Nexconn Pharmatechs Ltd
Hubei Jusheng Technology Co.,Ltd.




