| | 54063-53-5 Basic information More.. |
| Product Name: | Propafenone | | Synonyms: | WZ-884-643;β-Phenyl-2'-[3-(propylamino)-2-hydroxypropoxy]propiophenone;C07381;5-OH-Propafenone-D5;N-(carboxypheny)guanidine hydrochloride;1-(2-(2-Hydroxy-3-(propylamino);-3-phenylpropan-1-one;1-(2-(2-hydroxy-3-(propylamino)propoxy)phenyl)-3-phenyl-1-propanone | | CAS: | 54063-53-5 | | MF: | C21H27NO3 | | MW: | 341.44 | | EINECS: | 258-955-6 | | Mol File: | 54063-53-5.mol | 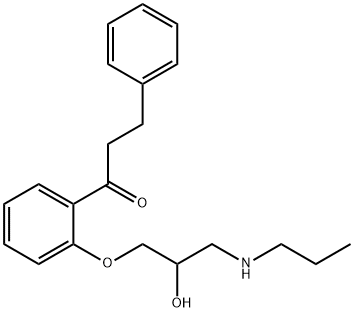 |
Use
Propafenone, 2-[2'-hydroxy-3-(propylamino)propoxy]-3-phenylpropiophenone (Rythmol), a classIC antiarrhythmic drug, contains a chiral center and is marketedas the racemic mixture. Therapy with the racemicmixture of propafenone produces effects that can be attributedto both (S) and (R) enantiomers. Although (R) and (S)enantiomers exert similarNa+channel–blocking effects, the(S) enantiomer also produces aβ-adrenergic blockade. As aresult, the (S) enantiomer is reported to be 40-fold morepotent than the (R) enantiomer as an antiarrhythmic agent.The enantiomers also display stereoselective dispositioncharacteristics. The (R) enantiomer is cleared more quickly.Hepatic metabolism is polymorphic and determined genetically.Ten percent of Caucasians have a reduced capacity tohydroxylate the drug to form 5-hydroxypropafenone. Thispolymorphic metabolism accounts for the interindividualvariability in the relationships between dose and concentrationand, thus, variability in the pharmacodynamic effects ofthe drug. The 5-hydroxy metabolites of both enantiomersare as potent as the parent compound in blocking Na+channels.Propafenone also depresses the slow inward current ofCa2+ions.
- Propafenone
-

- US $0.00-0.00 / kg
- 2025-01-03
- CAS:54063-53-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20tons
- Propafenone
-

- US $0.00 / KG
- 2024-12-27
- CAS:54063-53-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500kg/month
- Propafenone
-

- US $39.00-105.00 / mg
- 2024-11-19
- CAS:54063-53-5
- Min. Order:
- Purity: 99.69%
- Supply Ability: 10g
|
|
54063-53-5
Recommend Suppliers |
|