| | 2180-92-9 Basic information More.. |
| Product Name: | Bupivacaine | | Synonyms: | (.+/-.)-1-Butyl-2',6'-pipecoloxylidide;1-Butyl-2-(2,6-xylycarbamoyl)piperidine;1-Butyl-2',6'-pipecoloxylidide;1-butyl-2’,6’-pipecoloxylidide;1-butyl-n-(2,6-dimethylphenyl)-2-piperidinecarboxamid;Bupivacaine Base & HCL;Bupivacaine anhydrous;Bupivacaine (USP) | | CAS: | 2180-92-9 | | MF: | C18H28N2O | | MW: | 288.43 | | EINECS: | 218-553-3 | | Mol File: | 2180-92-9.mol | 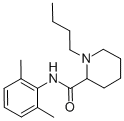 |
Use
Bupivacaine was synthesized simultaneously with mepivacainein 1957 but was at first overlooked because of the increasedtoxicity compared with mepivacaine. When themethyl on the cyclic amine of mepivacaine is exchanged fora butyl group the lipophilicity, potency and the duration ofaction all increase. Literature reports of cardiovascular toxicity,including severe hypotension and bradycardia, areabundant in the literature.91 Bupivacaine is highly bound toplasma proteins (95%), and thus the free concentration mayremain low until all of the protein binding sites are occupied.After that point, the plasma levels of bupivacaine rise rapidlyand patients may progress to overt cardiac toxicity withoutever showing signs of CNS toxicity. The cardiotoxicity ofbupivacaine is a result of its affinity to cardiac tissues and itsability to depress electrical conduction and predispose theheart to reentry types of arrhythmias. The cardiotoxicity ofbupivacaine was found to be significantly more prominentwith the “R” isomer, or the racemic mixture, thus the “S”stereoisomer is now on the market as levobupivacaine.
- Bupivacaine
-

- US $0.00-0.00 / kg
- 2025-04-18
- CAS:2180-92-9
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 800kg
- Bupivacaine
-

- US $0.00 / kg
- 2025-04-18
- CAS:2180-92-9
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1000
- Bupivacaine
-

- US $0.00-0.00 / KG
- 2025-04-16
- CAS:2180-92-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500
|
|
2180-92-9
Recommend Suppliers |
|