| Company Name: |
|
| Tel: |
|
| Email: |
ajay.malik@ap.umicore.com |
| Products Intro: |
Cas:13569-65-8
ProductName:Rhodium chloride, trihydrate
Purity: 99% | Package: 1 kg,5 kg, 10 kg,25kg and 1 MT
|
|
| | Rhodium Chloride Trihydrate Basic information |
| Product Name: | Rhodium Chloride Trihydrate | | Synonyms: | RHODIUM(III) CHLORIDE TRIHYDRATE (38% RH);Rhodium chloride (RhCl3), trihydrate;Triaquatrichlororhodium;RhodiuM chloride(RhCl3), trihydrate (8CI,9CI);chloride trihydrate;Rhodium (III) chloride cryohydrate;Rhodium(III) chloride trihydrate (38% Rh) for synthesis;RHODIUM TRICHLORIDE, HYDRATE | | CAS: | 13569-65-8 | | MF: | Cl3H6O3Rh | | MW: | 263.31 | | EINECS: | 682-140-8 | | Product Categories: | | | Mol File: | 13569-65-8.mol | 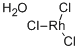 |
| | Rhodium Chloride Trihydrate Chemical Properties |
| Hazard Codes | Xn | | Risk Statements | 22-41 | | Safety Statements | 26-36/39 | | RIDADR | 3260 | | WGK Germany | WGK 1 slightly water endangering | | HazardClass | 8 | | PackingGroup | III | | HS Code | 28439090 | | Toxicity | LD50 orally in Rabbit: 753 mg/kg LD50 dermal Rabbit > 2000 mg/kg |
| | Rhodium Chloride Trihydrate Usage And Synthesis |
| Description | Rhodium trichloride is an odourless, red-brown to black crystalline powder. It is corrosive
to steel and aluminium. It is hygroscopic and absorbs moisture or water from the air. It is
incompatible with strong oxidising agents. On decomposition, rhodium trichloride emits
hydrogen chloride, carbon monoxide, carbon dioxide, and rhodium/rhodium oxides. | | Chemical Properties | Rhodium trichloride is a red-brown or black,
odorless solid or liquid | | Potential Exposure | Rhodium trichloride is used in hydro-
silylation, hydrogenation, carbonylation, oxidation, aryla-
tion. See also “Rhodium Metal.” In plating operations and
in catalyst preparation, the metal will be used as the
trichloride. | | Shipping | UN3260 Corrosive solid, acidic, inorganic, n.o.s.,
Hazard class: 8; Labels: 8-Corrosive material, Technical
Name Required. | | Incompatibilities | Sensitive to humidity. Incompatible with
oxidizers (chlorates, nitrates, peroxides, permanganates,
perchlorates, chlorine, bromine, fluorine, etc.); contact may
cause fires or explosions. Keep away from alkaline materi-
als, strong bases, strong acids, oxoacids, and epoxides. | | Waste Disposal | Recovery and reclaiming
wherever possible in view of high economic value. See
“Rhodium Metal.” |
| | Rhodium Chloride Trihydrate Preparation Products And Raw materials |
|