| Company Name: |
Shanghai Beiwanta Biotechnology Co., Ltd.
|
| Tel: |
021-67187366 19901745723 |
| Email: |
info@bwtlab.com |
| Products Intro: |
Cas:1216468-84-6
ProductName:13C6]-4-Iodonitrobenzene
Purity: 95+ | Package: 1mg;5mg;10mg;20mg
|
| Company Name: |
SynQuest Laboratories, Inc.
|
| Tel: |
904 462 0788 |
| Email: |
info@synquestlabs.com |
| Products Intro: |
Cas:636-98-6
ProductName:4-Iodonitrobenzene
Brand:SynQuest Laboratories | Product Number:4654-H-05
|
Iodonitrobenzene manufacturers
|
| | Iodonitrobenzene Basic information |
| Product Name: | Iodonitrobenzene | | Synonyms: | P-IODONITROBENZENE;P-NITROIODOBENZENE;4-IODONITROBENZENE;AURORA KA-6698;IODONITROBENZENE;1-IODO-4-NITROBENZENE | | CAS: | 30306-69-5 | | MF: | C6H4INO2 | | MW: | 249.01 | | EINECS: | 202-676-4 | | Product Categories: | | | Mol File: | 30306-69-5.mol | 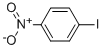 |
| | Iodonitrobenzene Chemical Properties |
| | Iodonitrobenzene Usage And Synthesis |
| Uses | 1-Iodo-4-nitrobenzene is a generic chemical reagent used in organic synthesis such as the stereoselective semihydrogenation of alkynes or in the formulation of new arylisoquinolones with anti-inflammatory activity. | | Preparation | Preparation of 1-iodo-4-nitrobenzene from 4-Nitroaniline.
Principle: Diazonium salts undergo a large number of reactions in which the diazo group is lost as molecular nitrogen and is replaced by variety of other groups (e.g. OH, I, Br, Cl, F, CN, NO2, H, SO2H, Ar) which become attached to the aromatic ring.
Reaction:
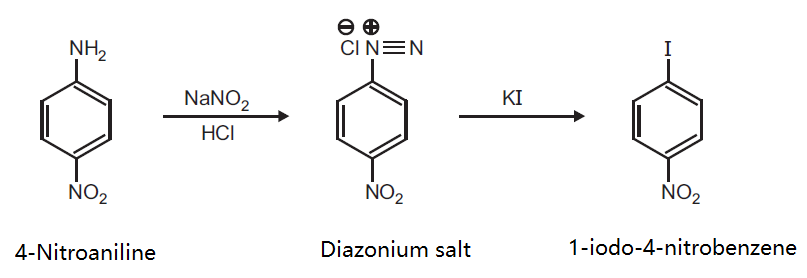
Procedure: Take 0.5 g p-nitro aniline, 0.41 ml conc. H2SO4 and 3 ml water in a conical flask. Stir it well and cool to 0-5°C. Add a cold solution of 0.25 g NaNO2 in 0.75 ml distilled water to the above solution. Filter the resultant cold solution, and add the filtrate with stirring to a solution of 1 g KI in 3 ml water taken in a beaker. Filter the product separated out and wash it with water. Dry the product, record the practical yield and re-crystallize it.
Re-crystallization: Dissolve the crude product in minimum amount of ethyl alcohol in a beaker by heating on a water bath. Filter the hot solution and cool the filtrate. The shiny brown crystals of p-iodo nitro benzene separate out. Filter, dry and record the melting point and TLC (using toluene as a solvent). | | Synthesis Reference(s) | Journal of the American Chemical Society, 83, p. 1928, 1961 DOI: 10.1021/ja01469a036
The Journal of Organic Chemistry, 33, p. 3670, 1968 DOI: 10.1021/jo01273a083 |
| | Iodonitrobenzene Preparation Products And Raw materials |
|