- Avibactam
-

- $0.00 / 1g
-
2024-03-12
- CAS:1192500-31-4
- Min. Order: 1g
- Purity: 98% HPLC
- Supply Ability: 1kg
- Avibactam
-

- $200.00 / 1kg
-
2023-06-26
- CAS:1192500-31-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg/Months
- Avibactam
-

- $200.00 / 10g
-
2022-11-23
- CAS:1192500-31-4
- Min. Order: 10g
- Purity: 99%
- Supply Ability: 1000t/month
|
| | AvibactaM Basic information |
| Product Name: | AvibactaM | | Synonyms: | AvibactaM;Sulfuric acid mono[(1R,2S,5R)-2-(aminocarbonyl)-7-oxo-1,6-diazabicyclo[3.2.1]oct-6-yl] ester;(E)-7-fluoro-3-methylhept-4-en-2-one;Avibactam free acid;Avibactam,(2S,5R)-7-Oxo-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]octane-2-carbox amide;(2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-ylhydrogensulfate;(2S,5R)-7-Oxo-6-(sulfooxy)-1,6-diazabicyclo[3.2.1]octane-2-carbox amide;AVIBACTAM FREE ACID (NXL-104 FREE ACID) | | CAS: | 1192500-31-4 | | MF: | C7H11N3O6S | | MW: | 265.24 | | EINECS: | 685-962-2 | | Product Categories: | | | Mol File: | 1192500-31-4.mol | 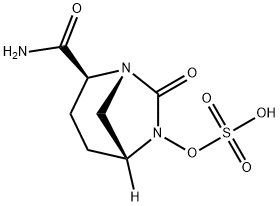 |
| | AvibactaM Chemical Properties |
| density | 1.85 | | storage temp. | Store at -20°C | | solubility | DMSO | | form | Powder | | pka | -4.59±0.18(Predicted) | | color | White to off-white |
| | AvibactaM Usage And Synthesis |
| Uses | Avibactam Butylammonium Salt is a novel β-lactamase inhibitor with a non-lactam structural scaffold. Avibactam irreversibly inhibits lactamase from Mycobacterium tuberculosis. | | Definition | ChEBI: A member of the class of azabicycloalkanes that is (2S,5R)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide in which the amino hydrogen at position 6 is replaced by a sulfooxy group. Used (in the form of its sodium salt)
n combination with ceftazidime pentahydrate for the treatment of complicated urinary tract infections including pyelonephritis. | | Enzyme inhibitor | This non-β-lactam inhibitor of β-lactamase (FW = 261.27 g/mol; CAS 1192500-31-4), also known as NLX104 and AVE1330A, shows a spectrum of action against Classes A and C β-lactamase as well as selected Class D β-lactamase, enzymes that often confer resistance to β-lactam antibiotics. By forming a high-affinity complex with its target enzymes, avibactam enhances the antibacterial activity of certain β-lactam drugs, such as ceftaroline. With over 1000 known β-lactamase, the action of avibactam is apt to depend on the invidual active-site interactions. Mode of Action: Acylation and deacylation rates have been measured for the clinically important enzymes CTX-M-15, KPC-2, E. cloacae AmpC, P. aeruginosa AmpC, OXA-10, and OXA-48. The efficiency of acylation (kon/Ki) varies across the enzyme spectrum, from 1.1 x 101 M–1 s –1 for OXA-10 to 1.0 x 105 M–1 s –1 for CTX-M-15. Inhibition of OXA-10 was shown to follow a reversible covalent mechanism (see below), and the acylated OXA-10 displayed the longest residence time for deacylation, with a half-life of greater than 5 days. The inhibited enzyme forms are stable to hydrolysis for all enzymes with the exception of KPC-2, which displays slow hydrolytic route that involved fragmentation of the acyl-avibactam complex. In the case of TEM-1 lactamase, avibactam slowly covalently acylates its target, and the acylated enzyme subsequently undergoes slow deacylation (koff = 0.045 min?1 ) regenerating avibactam intact. Use as a Combined Drug: Combination of avibactim with extended-spectrum cephalosporins or aztreonam shows promise in inhibiting Klebsiella pneumoniae (KP) isolates harboring carbapenemases, or KPCs. Given abundant experience with ceftazidime and the significant improvement avibactam provides against contemporary β-lactamase-producing Gram-negative pathogens, this combination will likely play a role in the treatment of complicated urinary tract infections (as monotherapy) and complicated intra-abdominal infections (in combination with metronidazole) caused (or suspected to be caused) by otherwise resistant pathogens, such as extended spectrum β-lactamase-, AmpC-, or Klebsiella pneumoniae carbapenemase producing Enterobacteriaceae and multidrug-resistant P. aeruginosa. |
| | AvibactaM Preparation Products And Raw materials |
| Raw materials | Avibactam INT 1-->(S)-ethyl 2-((tert-butoxycarbonyl)amino)-6-(dimethylhydrosulfinyl)-5-hydroxyhex-5-enoate-->Avibactam Impurity 15-->(S)-ethyl 4-(chloromethyl)-11,11-dimethyl-9-oxo-1-phenyl-2,10-dioxa-3,8-diazadodec-3-ene-7-carboxylate-->L-Norleucine, 6-chloro-N-[(1,1-dimethylethoxy)carbonyl]-5-[(phenylmethoxy)imino]-, phenylmethyl ester-->Benzyl (2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxylate ethanedioate-->2-Piperidinecarboxylic acid, 5-[(phenylmethoxy)imino]-, phenylmethyl ester, (2S)--->(2s,5r)-methyl 5-((benzyloxy)amino)piperidine-2-carboxylate oxalate-->(2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide-->(2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxamide-->BOC-L-PYROGLUTAMIC ACID BENZYL ESTER |
|