- Ipragliflozin
-

- $52.00 / 25mg
-
2025-04-30
- CAS:761423-87-4
- Min. Order:
- Purity: 99.97%
- Supply Ability: 10g
- Ipragliflozin
-

- $6.00 / 1kg
-
2025-04-28
- CAS:761423-87-4
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 1000
- Ipragliflozin
-

- $0.00 / 50g
-
2025-04-28
- CAS:761423-87-4
- Min. Order: 50g
- Purity: 99%
- Supply Ability: 15kg
|
| | Ipragliflozin Basic information |
| Product Name: | Ipragliflozin | | Synonyms: | Ipragliflozin, >=98%;IpragliflozinL-Proline;Ipragliflozin (ASP1941);Ipragliflozin impuity;(1S)-1,5-Anhydro-1-C-[3-[(1-benzothiophen-2-yl)methyl]-4-fluorophenyl]-D-glucitol;Ipragliflozin;ASP-1941/D-Glucitol, 1,5-anhydro-1-C-[3-(benzo[b]thien-2-ylMethyl)-4-fluorophenyl]-, (1S)-;Ipragliflozin, (1S)-1,5-Anhydro-1-C-[3-[(1-benzothiophen-2-yl)Methyl]-4-fluorophenyl]-D-glucitol | | CAS: | 761423-87-4 | | MF: | C21H21FO5S | | MW: | 404.45 | | EINECS: | | | Product Categories: | Inhibitors | | Mol File: | 761423-87-4.mol | 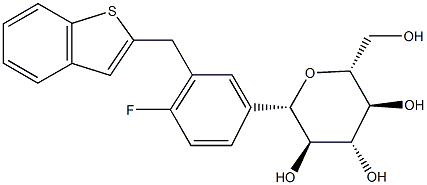 |
| | Ipragliflozin Chemical Properties |
| Melting point | 155-157°C | | Boiling point | 628.8±55.0 °C(Predicted) | | density | 1.452 | | storage temp. | Refrigerator | | solubility | DMSO (Slightly), Methanol (Slightly) | | pka | 13.27±0.70(Predicted) | | form | Solid | | color | White to Off-White |
| | Ipragliflozin Usage And Synthesis |
| Description | Ipragliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor (IC50 = 7.4 nM in CHO cells expressing the human cotransporter). It is selective for SGLT2 over SGLT1, SGLT3, SGLT4, SGLT5, and SGLT6 (IC50s = 1.9, 30.4, 15.9, 0.46, and 10.4 μM, respectively). Ipragliflozin (0.1-3 mg/kg) decreases plasma levels of insulin and glucose in an oral glucose tolerance test in a mouse model of diabetes induced by high-fat diet, streptozotocin (STZ; ), and nicotinamide . It decreases plasma and hepatic IL-6, TNF-α, chemokine (C-C motif) ligand 2 (CCL2), and C-reactive protein (CRP) levels in the same model when administered at a dose of 3 mg/kg per day for 28 days. | | History | Ipragliflozin L-proline was approved in Japan in January 2014 for the treatment of type 2 diabetes. The drug was discovered by Astellas Pharma and co-developed and marketed with Kotobuki Pharmaceutical and Merck Sharp Dohme as Suglat?. Similar to empagliflozin (XIII), ipragliflozin L-proline is a sodium-glucose 1956 A. C. Flick et al. / Bioorg. Med. Chem. 24 (2016) 1937–1980 co-transporter-2 inhibitor which prevents glucose reabsorption by excreting excess glucose in the urine. Ipragliflozin exhibits remarkable selectivity over SLGT-1 (>250x). | | Uses | Ipragliflozin is a potent and selective inhibitor of sodium-glucose cotransporter-2 (SGLT2) and can serve as a potential agent for the treatment of type 1 and type 2 diabetes. | | Definition | ChEBI: Ipragliflozin is a glycoside. | | Trade name | Suglat | | Synthesis | Commercial 5-bromo-2-fluorobenzaldehyde (123) was
subjected to nucleophilic attack upon subjection to lithiated
benzo[b]thiophene (124) to afford the dibenzylic alcohol 125 in
85% yield. This alcohol was then halogenated by means of thionyl
chloride in acetonitrile to give 126, which was followed by treatment
with sodium borohydride to give rise to 2-(5-bromo-2-fluorophenyl)-
1-benzothiophene (127), which was isolated by
crystallization from 2-propanol and methanol in 81% yield across
the two steps. Bromide 127 then underwent lithium¨Chalogen
exchange prior to exposure to 2,3,4,6-tetrakis-O-(trimethylsilyl)-
D-glucono-1,5-lactone (128) in toluene. Without workup, the
resulting mixture was treated with a solution of methanol and
HCl at 0 �C to give a globally desilylated a-glucopyranoside intermediate.
Subjection to acetic anhydride and 4-dimethylaminopyridine
furnished tetra-O-acetyl ipragliflozin (129) in 75% yield for
the 3 steps. Polyacetate 129 was then saponified using aqueous
sodium hydroxide and the product was crystallized from methanol
and water and subsequently treated with D-proline in ethanol to
furnish the desired product ipragliflozin D-proline (XVI) in 68%
yield. 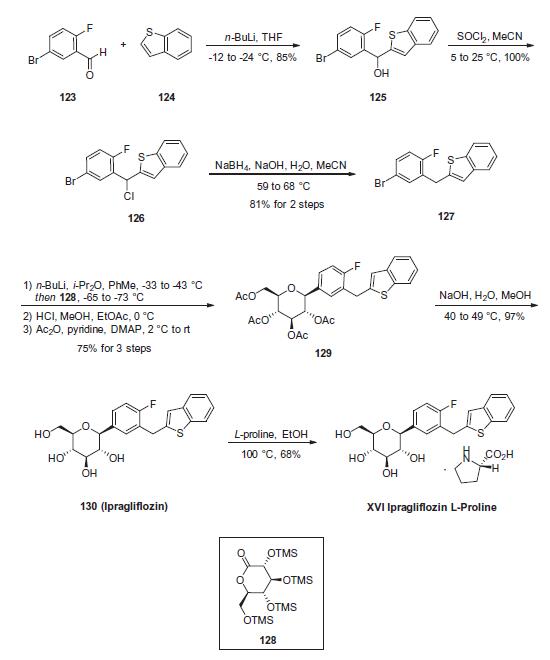
| | Mode of action | Ipragliflozin is a selective SGLT2 (sodium-glucose co-transporter 2) inhibitor discovered through research collaboration with Kotobuki Pharmaceutical Co., Ltd. SGLTs are membrane proteins that exist on the cell surface and transfer glucose into cells. SGLT2 is a subtype of the sodium-glucose co-transporters and plays a key role in the reuptake of glucose in the proximal tubule of the kidneys. Ipragliflozin reduces blood glucose levels by inhibiting the reuptake of glucose. |
| | Ipragliflozin Preparation Products And Raw materials |
|