BMS 201038-04 manufacturers
- BMS 201038-04 USP/EP/BP
-
- $1.10 / 1g
-
2021-08-17
- CAS:202914-84-9
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min
- Lomitapide Mesylate
-

- $1.00 / 1KG
-
2019-12-24
- CAS:202914-84-9
- Min. Order: 1KG
- Purity: Min98% HPLC
- Supply Ability: g/kg/ton
|
| | BMS 201038-04 Basic information |
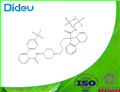
| Product Name: | BMS 201038-04 | | Synonyms: | BMS 201038-04;LoMitapide Mesylate;BMS 201038 methanesulfonic acid salt;AEGR 733 Mesylate;AEGR733 Mesylate;AEGR-733 Mesylate;BMS 201038 Mesylate;BMS201038 Mesylate | | CAS: | 202914-84-9 | | MF: | C40H41F6N3O5S | | MW: | 789.8260592 | | EINECS: | | | Product Categories: | Inhibitors | | Mol File: | 202914-84-9.mol |  |
| | BMS 201038-04 Chemical Properties |
| storage temp. | Inert atmosphere,Room Temperature | | solubility | DMSO:100.0(Max Conc. mg/mL);126.61(Max Conc. mM)
Ethanol:100.0(Max Conc. mg/mL);126.61(Max Conc. mM) | | form | Solid | | color | White to off-white | | CAS DataBase Reference | 202914-84-9 |
| | BMS 201038-04 Usage And Synthesis |
| Description | Lomitapide is an orally active microsomal triglyceride transfer
protein (MTP) inhibitor for the treatment of hypercholesterolemia.
The drug was developed by Aegerion
Pharmaceuticals Inc. and licensed to Bristol–Myers Squibb Co.
and the University of Pennsylvania. Lomitapide effectively lowered
LDL–cholesterol, both as a single agent and in combination
with commonly prescribed lipid-lowering therapies. Sold under
the trade name Juxtapid�, the drug offers a new treatment option
to patients who cannot tolerate statin therapy or who experience
insufficient LDL–cholesterol reduction with the currently available
therapies, such as patients with homozygous familial hypercholesterolemia
caused by mutations in the LDLR gene. | | Definition | ChEBI: A methanesulfonate (mesylate) salt prepared from equimolar amounts of lomitapide and methanesulfonic acid. Used as a complement to a low-fat diet and other lipid-lowering treatments in patients with homozygous familial hypercholesterolemia. | | Synthesis | Commercial 9H-fluorene-9-carboxylic acid (105) was alkylated
with 1,4-dibromobutane in the presence of n-butyl lithium in
THF to give 9-(4-bromobutyl)-9H-fluorene-9-carboxylic acid
(106) in 85% yield. Next, activation of the acid as the acid chloride
followed by coupling with (2,2,2-trifluoroethylamine) provided
amide 107 in 71% yield for the two-step sequence. Displacement
of the terminal bromide with the appropriate 4-carbamoyl
piperidine followed by removal of the Boc group furnished piperidinyl
fluorine 108 in high yield. Amine 108 was then reacted with
the acid chloride derived from acid 109 (derived from the Suzuki
coupling of boronic acid 110 and o-iodobenzoic acid 111) to give
lomitapide, and this was followed by salt formation with methanesulfonic
acid to afford lomitapide mesylate (XIV). 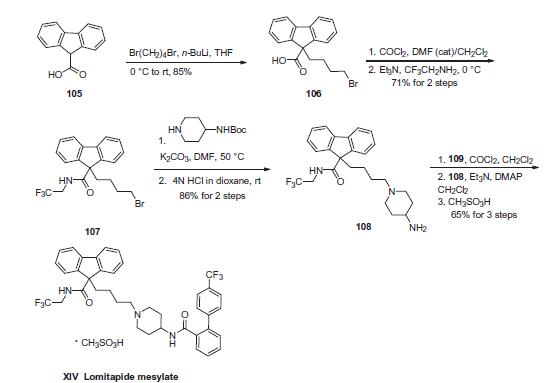
|
| | BMS 201038-04 Preparation Products And Raw materials |
|