| Company Name: |
TaiChem Taizhou Limited
|
| Tel: |
052386810091 |
| Email: |
zcwy9518@yeah.net |
| Products Intro: |
Product Name:Rac-(4aR,8aR)-decahydroquinoline
CAS:10343-99-4
Purity:95% Package:1G;2G;5G
|
| Company Name: |
Jiangsu yize medical technology co. LTD
|
| Tel: |
18962082606 |
| Email: |
3394205705@qq.com |
| Products Intro: |
Product Name:cis-1,2,3,4,4a,5,6,7,8,8a-decahydroquinoline
CAS:10343-99-4
Package:1kg
|
| Company Name: |
Shaanxi Dideu Newmaterial Co., Ltd.
|
| Tel: |
029-87576359 15353716720 |
| Email: |
1039@dideu.com |
| Products Intro: |
Product Name:cis-decahydroquinoline
CAS:10343-99-4
Purity:80% Package:25kg
|
| Company Name: |
Combi-Blocks Inc.
|
| Tel: |
858 635 8950 |
| Email: |
sales@combi-blocks.com |
| Products Intro: |
Product Name:Rac-(4aR,8aR)-decahydroquinoline
CAS:10343-99-4
|
|
| | cis-decahydroquinoline Basic information |
| Product Name: | cis-decahydroquinoline | | Synonyms: | cis-decahydroquinoline;Quinoline, decahydro-, (4aR,8aR)-rel-;Rac-(4aR,8aR)-decahydroquinoline;cis-1,2,3,4,4a,5,6,7,8,8a-decahydroquinoline | | CAS: | 10343-99-4 | | MF: | C9H17N | | MW: | 139.24 | | EINECS: | 2337525 | | Product Categories: | | | Mol File: | 10343-99-4.mol | 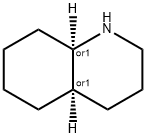 |
| | cis-decahydroquinoline Chemical Properties |
| Melting point | -40°C | | Boiling point | 245.24°C (rough estimate) | | density | 0.9426 | | refractive index | 1.4926 (estimate) | | pka | 10.85±0.10(Predicted) |
| | cis-decahydroquinoline Usage And Synthesis |
| Purification Methods | It is available as a cis-trans-mixture (b 70-73o/10mm, Aldrich, ~ 18% cis-isomer [2051-28-7]), but the isomers can be separated by fractionating in a spinning band column (1~1.5 metre, type E) at atmospheric pressure and collecting 2mL fractions with a distillation rate of 1 drop in 8-10seconds. The lower boiling fraction solidifies and contains the trans-isomer (see below, m 48o). The higher boiling fraction b 207-208o/708mm remains liquid and is mostly pure cis-isomer. This is reacted with PhCOCl and M aqueous NaOH to yield the N-benzoyl derivative m 96o after recrystallisation from pet ether (b 80-100o). It is hydrolysed with 20% aqueous HCl by refluxing overnight. PhCO2H is filtered off, the filtrate is basified with 5M aqueous NaOH and extracted with Et2O. The dried extract (Na2SO4) is saturated with dry HCl gas, and the cis-decahydroquinoline hydrochloride which separates has m 222-224o after washing with Et2O and drying at 100o; and has IR (KBr) 2900, max 2780, 2560, 1580, 1445, 1432, 1403, 1165, 1080, 1036, 990, 867 cm -1. The free base is obtained by dissolving the hydrochloride salt in 5M aqueous NaOH, extracting with Et2O and drying the extract (Na2SO4), evaporating and distilling the residue; it has IR (film) 2900, 2840, 2770, 1445, 1357, 1330, 1305, 1140, max 1125, 1109, 1068, 844 cm-1 . The 1H NMR in CDCl3 is characteristically different from that of the trans-isomer. [Armarego J Chem Soc (C) 377 1967, Hückel & Stepf Justus Liebigs Ann Chem 453 163 1927, Bailey & McElvain J Am Chem Soc 52 4013 1930, Beilstein 20 H 157, 20 I 35, 20 II 72-73, 20 III/IV 2017.] |
| | cis-decahydroquinoline Preparation Products And Raw materials |
|