|
|
| | 2-Thiopheneacetic acid, alpha-hydroxy-alpha-2-thienyl-, (1R,3R,5R)-6,6 ,9-trimethyl-9-azabicyclo(3.3.1)non-3-yl ester, hydrochloride, rel- Basic information |
| Product Name: | 2-Thiopheneacetic acid, alpha-hydroxy-alpha-2-thienyl-, (1R,3R,5R)-6,6 ,9-trimethyl-9-azabicyclo(3.3.1)non-3-yl ester, hydrochloride, rel- | | Synonyms: | 2-Thiopheneacetic acid, alpha-hydroxy-alpha-2-thienyl-, (1R,3R,5R)-6,6 ,9-trimethyl-9-azabicyclo(3.3.1)non-3-yl ester, hydrochloride, rel-;2-Thiopheneacetic acid, alpha-hydroxy-alpha-2-thienyl-, 6,6,9-trimethyl-9-azabicyclo(3.3.1)non-3-yl ester, hydrochloride, exo-;Glycolic acid, di-2-thienyl-, 6,6,9-trimethyl-9-azabicyclo(3.3.1)non-3-yl ester, hydrochloride, exo-;(8,8,9-trimethyl-9-azabicyclo[3.3.1]non-3-yl) 2-hydroxy-2,2-dithiophen -2-yl-acetate hydrochloride | | CAS: | 38738-59-9 | | MF: | C21H28ClNO3S2 | | MW: | 0 | | EINECS: | | | Product Categories: | | | Mol File: | 38738-59-9.mol | 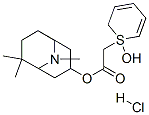 |
| | 2-Thiopheneacetic acid, alpha-hydroxy-alpha-2-thienyl-, (1R,3R,5R)-6,6 ,9-trimethyl-9-azabicyclo(3.3.1)non-3-yl ester, hydrochloride, rel- Chemical Properties |
| | 2-Thiopheneacetic acid, alpha-hydroxy-alpha-2-thienyl-, (1R,3R,5R)-6,6 ,9-trimethyl-9-azabicyclo(3.3.1)non-3-yl ester, hydrochloride, rel- Usage And Synthesis |
| Originator | Pentona, Tanabe ,Japan ,1978 | | Manufacturing Process | A mixture of 1.0 g of 6,6,9-trimethyl-9-azabicyclo[3.3.1]nonan-3β-ol, methyl α,α-di-(2-thienyl)-glycolate and 30 mg of metallic sodium is heated at 80°C to 90°C for about 2 hours under reduced pressure. After cooling, ether is added to the reaction mixture. The mixture is extracted with 10% hydrochloric acid. The aqueous layer is alkalified with sodium carbonate and reextracted with ethyl acetate. The extract is washed with water, dried and concentrated to dryness. The residue thus obtained is treated with hydrogen chloride by conventional manner. 2.0 g of the α,α-di-(2-thienyl)glycolate of 6,6,9- trimethyl-9-azabicyclo[3.3.1]nonan-3β-ol hydrochloride are obtained. Yield 83%. | | Therapeutic Function | Antiparkinsonian |
| | 2-Thiopheneacetic acid, alpha-hydroxy-alpha-2-thienyl-, (1R,3R,5R)-6,6 ,9-trimethyl-9-azabicyclo(3.3.1)non-3-yl ester, hydrochloride, rel- Preparation Products And Raw materials |
|