|
|
| | (8ξ,9ξ,10ξ,11ξ,12ξ,13ξ,14ξ)-2-(Tetrahydro-4-methyl-5-oxofuran-2-yl)stenine Basic information |
| Product Name: | (8ξ,9ξ,10ξ,11ξ,12ξ,13ξ,14ξ)-2-(Tetrahydro-4-methyl-5-oxofuran-2-yl)stenine | | Synonyms: | (8ξ,9ξ,10ξ,11ξ,12ξ,13ξ,14ξ)-2-(Tetrahydro-4-methyl-5-oxofuran-2-yl)stenine;Stemonine;Stemonine【C22 alkaloid】;Furo[2,3-h]pyrrolo[3,2,1-jk][1]benzazepin-10(2H)-one, 8-ethyldodecahydro-11-methyl-2-(tetrahydro-4-methyl-5-oxo-2-furanyl)- | | CAS: | 20460-41-7 | | MF: | C22H33NO4 | | MW: | 375.5 | | EINECS: | | | Product Categories: | | | Mol File: | 20460-41-7.mol | 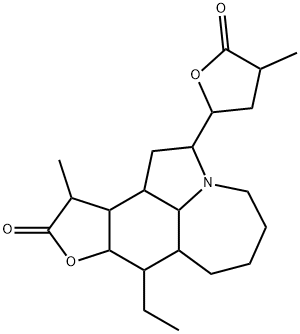 |
| | (8ξ,9ξ,10ξ,11ξ,12ξ,13ξ,14ξ)-2-(Tetrahydro-4-methyl-5-oxofuran-2-yl)stenine Chemical Properties |
| | (8ξ,9ξ,10ξ,11ξ,12ξ,13ξ,14ξ)-2-(Tetrahydro-4-methyl-5-oxofuran-2-yl)stenine Usage And Synthesis |
| Description | This base occurs with Stemonidine (q.v.) in Stemona ovata Nakai. It is laevo_x0002_rotatory with [α]16D - 113.84° and crystallizes as colourless prisms. It gives
crystalline salts such as the hydrochloride, m.p. 302°C (dec.) and is a secondary
base. With methyl iodide it forms methylstemonine methiodide, m.p. 276°C
(dec. ). | | References | Suzuki., J. Pharm. Soc., Japan, 49, 78 (1929)
Suzuki., ibid, 51,43 (1931)
Suzuki., ibid, 54, 101 (1934) |
| | (8ξ,9ξ,10ξ,11ξ,12ξ,13ξ,14ξ)-2-(Tetrahydro-4-methyl-5-oxofuran-2-yl)stenine Preparation Products And Raw materials |
|