| Company Name: |
BOC Sciences
|
| Tel: |
1-631-485-4226; 16314854226 |
| Email: |
info@bocsci.com |
| Products Intro: |
Product Name:Nintedanib Impurity E (Intedanib Impurity E)
CAS:1610881-60-1
Purity:> 95% Remarks:An impurity of Nintedanib, which is a potent triple angiokinase inhibitor for VEGFR1/2/3, FGFR1/2/3
|
| Company Name: |
Nanjing XiZe Biotechnology CO., Ltd.
|
| Tel: |
025-66023220 0086-15250997978 |
| Email: |
1511893459@qq.com |
| Products Intro: |
Product Name:Nintedanib Impurity E (Intedanib Impurity E)
Purity:95% HPLC Package:10mg;25mg;50mg;100mg;250mg;500mg;1g
|
| Company Name: |
Lynnchem
|
| Tel: |
86-(0)29-85992781 17792393971 |
| Email: |
info@lynnchem.com |
| Products Intro: |
Product Name:Nintedanib Impurity E (Intedanib Impurity E)
CAS:1610881-60-1
Purity:95% Package:5mg;25mg
|
| Company Name: |
Novachemistry
|
| Tel: |
44-20819178-90 02081917890 |
| Email: |
info@novachemistry.com |
| Products Intro: |
Product Name:Nintedanib Impurity E (Intedanib Impurity E)
Purity:95% Package:5mg;25mg
|
| Company Name: |
Shenzhen ruikai de biotechnology co., ltd.
|
| Tel: |
0755-61348847 17820674378 |
| Email: |
2682116078@qq.com |
| Products Intro: |
Product Name:Nintedanib Impurity E
(Intedanib Impurity E)
CAS:1610881-60-1
Purity:98%HPLC Package:10mg;25mg;50mg;100mg
|
|
| | Nintedanib Impurity E (Intedanib Impurity E) Basic information |
| Product Name: | Nintedanib Impurity E (Intedanib Impurity E) | | Synonyms: | Nintedanib Impurity E (Intedanib Impurity E);Nintedanib impuritiesE;N-Methyl-2-(4-methylpiperazin-1-yl)-N-(4-(((2-oxoindolin-3-ylidene)(phenyl)methyl)amino)phenyl)acetamide;1-Piperazineacetamide, N-[4-[[(1,2-dihydro-2-oxo-3H-indol-3-ylidene)phenylmethyl]amino]phenyl]-N,4-dimethyl- | | CAS: | 1610881-60-1 | | MF: | C29H31N5O2 | | MW: | 481.6 | | EINECS: | | | Product Categories: | | | Mol File: | 1610881-60-1.mol | 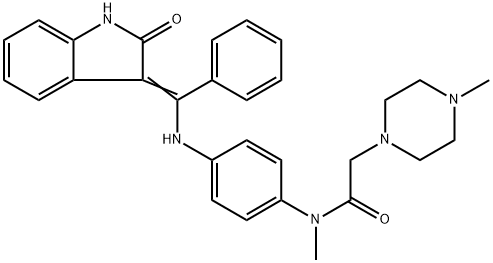 |
| | Nintedanib Impurity E (Intedanib Impurity E) Chemical Properties |
| Boiling point | 695.9±55.0 °C(Predicted) | | density | 1.259±0.06 g/cm3(Predicted) | | pka | 11.57±0.20(Predicted) |
| | Nintedanib Impurity E (Intedanib Impurity E) Usage And Synthesis |
| | Nintedanib Impurity E (Intedanib Impurity E) Preparation Products And Raw materials |
|