|
ChemicalBook Optimization Suppliers |
|
| 化学名: | N,N-ジエチルヒドロキシルアミン | | 英語化学名: | N,N-Diethylhydroxylamine | | 别名: | N,N-DIETHYLHYDROXYLAMINE, TECH;N,N-Diethylhydroxyla;DiethylhydroxylaMine (DEHA85);(D.E.H.A )N.N-DIETHYLHYDROXYLAMINE;N,N-DiethylhydroxylaMine, 97% 5GR;N,N-DiethylhydroxylaMine;N-ethyl-N-hydroxyethanaMine sulfate;N,N-DIETHYLHYDROXYLAMINE FOR SYNTHESIS | | CAS番号: | 3710-84-7 | | 分子式: | C4H11NO | | 分子量: | 89.14 | | EINECS: | 223-055-4 | | カテゴリ情報: | Hydroxylamines;Hydroxylamines (N-Substituted);Nitrogen Compounds;Organic Building Blocks;Building Blocks;Chemical Synthesis;Nitrogen Compounds;Organic Building Blocks;Amine series;Piperidines ,Homopiperidines;FINE Chemical & INTERMEDIATES;3710-84-7 | | Mol File: | 3710-84-7.mol | 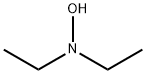 |
| 融点 | −26-−25 °C(lit.) | | 沸点 | 125-130 °C | | 比重(密度) | 0.902 g/mL at 25 °C | | 蒸気密度 | 3.07 (vs air) | | 蒸気圧 | 4 mm Hg ( 0 °C) | | 屈折率 | n20/D 1.420(lit.) | | 闪点 | 115 °F | | 貯蔵温度 | Store below +30°C. | | 酸解離定数(Pka) | 14.19±0.69(Predicted) | | 外見 | Liquid | | 色 | Clear | | 比重 | 0.867 | | 爆発限界(explosive limit) | 1.9-10%(V) | | 水溶解度 | soluble | | Sensitive | Hygroscopic | | BRN | 1731349 | | 暴露限界値 | ACGIH: TWA 2 ppm | | 安定性: | Stable. Hygroscopic. Flammable. Incompatible with strong oxidizing agents, strong acids. | | InChIKey | FVCOIAYSJZGECG-UHFFFAOYSA-N | | LogP | 0.5 at 23℃ | | CAS データベース | 3710-84-7(CAS DataBase Reference) | | NISTの化学物質情報 | Ethanamine, N-ethyl-N-hydroxy-(3710-84-7) | | EPAの化学物質情報 | Diethylhydroxylamine (3710-84-7) |
| | N,N-ジエチルヒドロキシルアミン Usage And Synthesis |
| 外観 | 無色~黄褐色, 澄明の液体 | | 溶解性 | エタノールに極めて溶けやすく、水に溶けやすい。 | | 用途 | 重合停止剤(スチレン-ブタジエンゴム等)、安定剤(スチレンモノマー)、 空気酸化防止剤(ラッカー、ニス等)、写真現像薬。 | | 化学的特性 | liquid | | 使用 | N,N-Diethylhydroxylamine may be employed as a ligand in the preparation of unsymmetric mixed ligand oxadiazoline and/or imine platinum complexes. It may be used in the synthesis of organometallic clusters of mixed hydrazide/hydroxylamide clusters of zinc. | | 使用 | N,N-Diethylhydroxylamine has the following various uses:
1.As a vinyl monomer, used in efficient inhibitor agent of conjugate olefins.
2.In liquid or gas phase, if end gather seed is existed,it can be used as inhibitors of end gather.
3.It's a excellent end agent in process of Emulsion-polymerized styrene butadiene rubber.
4.It's a antioxidants of the unsaturated oil and resin.
5.In the environmental protection,it’s good photochemical smoke inhibitors.
6.It's used as corrosion inhibitors in the equipment of the boiler feed water and steam heat exchange.
7.It's used as antioxidant in photography screens potions. | | 使用 | Diethylhydroxylamine has been suggested as a stabilizer for color formation for monoalkylphenols and phenolic antioxidants. It has also been reported to stabilize emulsions used in the latex industry and for Spandex rubber, as well as a reducing agent for quinones and a monomer stabilizer or inhibitor. | | 一般的な説明 | The product is 85wt.% solution of N,N-diethylhydroxylamine. The reaction of N,N-diethylhydroxylamine with tert-butylhydroperoxide has been studied. It participates in the preparation of symmetrical and an isomeric mixture of unsymmetrical phthalocyanines. It undergoes degradation on exposure to radiation and affords light hydrocarbons. Qualitative and quantitative analyses of the produced hydrocarbons have been reported. It also participates in the conversion of quinones and quinonemonosulfonimide to the corresponding hydroquinones and sulfonylaminophenols, respectively. | | 健康への影響 | The effects of diethylhydroxylamine (DEHA), a potent free-radical scavenger, on lipid peroxidation of rat liver microsomes were investigated in vitro. DEHA strongly inhibited ascorbate-dependent nonenzymatic microsomal lipid peroxidation. It also completely inhibited nonenzymatic lipid peroxidation of heat-denatured microsomes, indicating that inhibition is protein-independent. DEHA only moderately inhibited NADPH-dependent enzymatic microsomal lipid peroxidation. | | reaction suitability | reagent type: reductant | | 安全性プロファイル | Poison by skin contact.
Moderately toxic by ingestion and
intraperitoneal routes. Experimental
reproductive effects. Human mutation data
reported. When heated to decomposition it
emits toxic fumes of NOx. See also
AMINES. |
|