|
|
| | bepridil Basic information |
| Product Name: | bepridil | | Synonyms: | N-Benzyl-α-(isobutoxymethyl)-N-phenyl-1-pyrrolidineethanamine;1-Pyrrolidineethanamine, alpha-((2-methylpropoxy)methyl)-N-phenyl-N-(phenylmethyl)-;Einecs 256-384-7 | | CAS: | 49571-04-2 | | MF: | C24H34N2O | | MW: | 366.53956 | | EINECS: | 256-384-7 | | Product Categories: | | | Mol File: | 49571-04-2.mol | 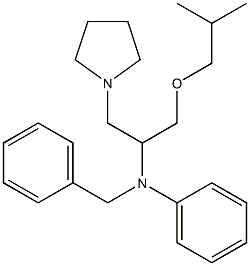 |
| | bepridil Chemical Properties |
| | bepridil Usage And Synthesis |
| Originator | Cordium,Riom,France,1981 | | Manufacturing Process | The first step involves the preparation of 1-(3-isobutoxy-2-chloro)propyl pyrrolidine as an intermediate. 345 ml of thionyl chloride dissolved in 345 ml
of chloroform are added, drop by drop, to 275 g of 1-(3-isobutoxy-2-
hydroxy)propyl pyrrolidine dissolved in 350 ml of chloroform, while
maintaining the temperature at approximately 45°. The reaction mixture is
heated to reflux until gas is no longer evolved. The chloroform and the excess
of thionyl chloride are removed under reduced pressure. The residue is poured
on to 400 g of crushed ice. The reaction mixture is rendered alkaline with
soda and the resulting mixture is extracted twice with 250 ml of diethyl ether.
The combined ethereal extracts are dried over anhydrous sodium sulfate.
After evaporation of the solvent the residue is distilled under reduced
pressure. 220 g of product are obtained having the following properties:
boiling point = 96°C/3 mm, nD24 = 1.4575.
The final product is prepared as follows. 23.4 g of sodium amide is added little
by little to a solution of 92 g of N-benzylaniline in 500 ml of anhydrous
xylene. The reaction mixture is then heated at 130°-135°C for 6 hours.
While maintaining the temperature at 110°C, 110 g of the product of the first
step dissolved in 150 ml of xylene is added and the product heated for 6
hours at 120°C.
The product having been allowed to cool to ambient temperature, 200 ml of
cold water are added. The organic phase is separated and extracted with an
aqueous solution of hydrochloric acid.
After twice washing with 100 ml of diethyl ether, the aqueous phase is made
alkaline with 50% caustic soda solution. The liberated base is twice extracted
with 150 ml of diethyl ether. After the ether has been evaporated, the residue
is distilled under reduced pressure and has a boiling point of 184°C/0.1 mm,
nD20 = 1.5538. 77 g of the pure base in the form of a viscous liquid is thus
obtained. The hydrochloride, which is prepared in conventional manner, has a
melting point of 128°C. | | Therapeutic Function | Antianginal |
| | bepridil Preparation Products And Raw materials |
|