|
|
| | MIPS-521 Basic information |
| Product Name: | MIPS-521 | | Synonyms: | MIPS-521;5-bis(trifluoromethyl)phenyl]thiophen-3-yl}(4-chlorophenyl)methanone;{2-Amino-4-[3,5-bis(trifluoromethyl)phenyl]thiophen-3-yl}(4-chlorophenyl)methanone;A1AR,inhibit,MIPS-521,pain,Adenosine Receptor,MIPS 521,MIPS521,Inhibitor,P1 receptor;Methanone, [2-amino-4-[3,5-bis(trifluoromethyl)phenyl]-3-thienyl](4-chlorophenyl)-;MIPS521, 10 mM in DMSO | | CAS: | 1146188-19-3 | | MF: | C19H10ClF6NOS | | MW: | 449.8 | | EINECS: | | | Product Categories: | | | Mol File: | 1146188-19-3.mol | 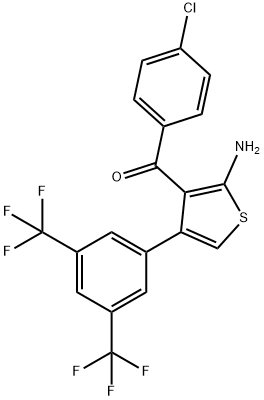 |
| | MIPS-521 Chemical Properties |
| Melting point | 178-180 °C(Solv: ligroine (8032-32-4)) | | Boiling point | 516.3±50.0 °C(Predicted) | | density | 1.481±0.06 g/cm3(Predicted) | | storage temp. | Store at -20°C | | solubility | DMSO : 25 mg/mL (55.58 mM; ultrasonic and warming and heat to 60°C) | | pka | -2.49±0.10(Predicted) | | form | Solid | | color | Light yellow to green yellow |
| | MIPS-521 Usage And Synthesis |
| Biological Activity | MIPS521 is a positive allosteric modulator of adenosine A1 receptor (A1AR). MIPS521 also has a lower A1R allosteric affinity (pKB=4.95). MIPS521 exhibits pain-relieving effects in vivo[1][2].
MIPS521 (compound 13o) (3-10 μM) improves the ability of R-PIA to promote A1AR-mediated ERK1/2 phosphorylation[1].MIPS521 (0.3-30 μM; pretreament for 10 min, co-treatment for 30 min) produces a concentration-dependent potentiation of signalling by ADO in an inhibition of cAMP assay (expressed as a percentage of the inhibition of 3 μM forskolin-mediated cAMP) in CHO cells[2].
MIPS521 (1-30 μg in 10 μL; intrathecal administration) reverses mechanical hyperalgesia in rats, promoting robust antinociception[2].MIPS521 (10 μg in 10 μL; intrathecal administration) significantly reduces spontaneous pain in a conditioned place preference model[2].MIPS521 (1-30 μg in 10 μL; intrathecal administration) reduces eEPSCs in spinal cord from nerve-injured rats, with a pEC50 of 6.9. The maximum MIPS521-induced decrease in synaptic current amplitude is significantly greater in nerve-injured rats than in sham surgery controls[2]. | | References | [1]. Aurelio L, et, al. Allosteric modulators of the adenosine A1 receptor: synthesis and pharmacological evaluation of 4-substituted 2-amino-3-benzoylthiophenes. J Med Chem. 2009 Jul 23;52(14):4543-7.
[2]. Draper-Joyce CJ, et, al. Positive allosteric mechanisms of adenosine A 1 receptor-mediated analgesia. Nature. 2021 Sep;597(7877):571-576. |
| | MIPS-521 Preparation Products And Raw materials |
|