- Alvimopan Dihydrate
-

- $0.00 / 1g
-
2024-09-06
- CAS:170098-38-1
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 50kg/Month
|
| | 2-[[(2S)-2-benzyl-3-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperi dyl]propanoyl]amino]acetic acid dihydrate Basic information |
| Product Name: | 2-[[(2S)-2-benzyl-3-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperi dyl]propanoyl]amino]acetic acid dihydrate | | Synonyms: | 2-[[(2S)-2-benzyl-3-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperi dyl]propanoyl]amino]acetic acid dihydrate;[[(2S)-2-[[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]methyl]-3-phenylpropanoyl]amino]acetic acid dehydrate;(((2S)-2-(((3R,4R)-4-(3-Hydroxyphenyl)-3,4-dimethylpiperidin-1-yl)methyl)-3-phenylpropanoyl)amino)acetic acid dihydrate;156053-89-3 (Anhydrous);Alvimopan hydrate;Entereg;Glycine, N-((2S)-2-(((3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl)methyl)-1-oxo-3-phenylpropyl)-, dihydrate;Glycine, N-(2-((4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl)methyl)-1-oxo-3-phenylpropyl)-,dihydrate, (3R-(1(S*),3-alpha,4-alpha))- | | CAS: | 170098-38-1 | | MF: | C25H36N2O6 | | MW: | 460.57 | | EINECS: | | | Product Categories: | API | | Mol File: | 170098-38-1.mol | ![2-[[(2S)-2-benzyl-3-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperi dyl]propanoyl]amino]acetic acid dihydrate Structure](CAS/GIF/170098-38-1.gif) |
| | 2-[[(2S)-2-benzyl-3-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperi dyl]propanoyl]amino]acetic acid dihydrate Chemical Properties |
| Melting point | 210-213° | | alpha | D25 +51.8° (c = 1.0 in DMSO) | | storage temp. | 2-8°C | | solubility | Soluble in DMSO | | form | Powder | | color | White to off-white |
| | 2-[[(2S)-2-benzyl-3-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperi dyl]propanoyl]amino]acetic acid dihydrate Usage And Synthesis |
| Description | Alvimopan is a μ-opioid receptor antagonist approved in
the U.S. in May 2008 for the treatment of post-operative
ileus (POI) – a temporary dysfunction of the gastrointestines.
Alvimopan does not penetrate the central nervous system
(CNS) and acts as a peripheral antagonist. The molecule inhibits
the negative effects of opioids on the gastrointestinal
(GI) system without inhibiting the desired analgesic effects
of CNS penetrant opioids. | | Uses | Treatment of opioid-induced bowel dysfunction, opioidinduced
nausea and vomiting, postoperative ileus, idiopathic
constipation, and irritable bowel syndrome (peripherally
restricted mu opioid receptor antagonist). | | Synthesis | Several synthetic routes have been disclosed, and the
process route is described in the scheme. This route was performed on kilogram scale and no yields were reported
beyond the generation of compound 8. 3-Bromophenol 1
was treated with isopropyl bromide and potassium carbonate
at 60-65 ??C for 16 h to give 3-isopropyoxy bromobenzene 2.
Bromide 2 was added to a suspension of Mg turnings in THF
at 40-60 ??C generating the corresponding Grignard reagent
to which a solution of 1,3-dimethylpiperidone 3 in THF was
added as four separate fractions over a period of 2 h. Upon
completion, the reaction mixture was quenched with aqueous
ammonium chloride, the product was extracted into heptane
and crystallized out of solution and was isolated by filtration
to provide a cis-(?à) enriched mixture of piperidone alcohol 4
in 97% purity. This mixture was recrystallized from heptane
to afford exclusively the cis-(?à) piperidone 4 in 97% purity
and 66% yield. Piperidone alcohol 4 was treated with ethylchloroformate
and triethyl amine at 0??C and warmed to
room temperature over 3 h. The resulting ethylcarbonate was
resolved via classical resolution with (+)-di-p-toluyl-Dtartaric
acid and then recrystallized from ethanol to give 5 in
99% purity and 99.5% ee. The conversion of 5 to 3,4-trans
dimethyl piperidine 8 followed the sequence described by
Werner, et. al. as no experimental was disclosed in the process
patent for this sequence. The (+)-DTTA salt 5
was treated with sodium hydroxide to liberate the free base
which then underwent thermal elimination of the carbonate
at 190 ??C in decalin to give the desired trisubstituted olefin 6
in 92% yield. Treatment of piperidine 6 with n-BuLi followed
by addition of dimethyl sulfate at -50 ??C gave the desired
3,4-trans-dimethyl enamine 7. Due to the reactivity of
the dimethyl sulfate, only one equivalent was used and the
reaction had to be quenched into aqueous ammonium hydroxide
to avoid N-methylation. The crude enamine 7 was
reduced with sodium borohydride and purified by crystallization
with (+)-DTTA, giving (+)-DTTA salt 8 in 65% overall
yield from 5. Additionally, the crystallization provided 8
with less than 1% impurities and 98.8% ee. The free base of
8 was liberated upon treatment with sodium hydroxide and
reacted with phenyl chloroformate at 80-85 ??C, to effectively
demethylate the nitrogen. The resulting crude phenylcarbamate
9 was refluxed in HBr/acetic acid for 18 h to simultaneously
cleave the isopropyl ether and carbamate protecting
groups to give the aminophenol 10, which was precipitated
out of solution and collected by filtration. Amine 10 was then treated with methylacrylate (11) in THF at 40-45 ??C for
18-19 h to give the intermediate 12, which was transferred
directly into a solution of LDA. A solution of benzyl bromide
in THF was added to the enolate of 12 at -20 ??C and
upon complete benzylation, 13 was isolated as its HCl salt.
Ester 13 was hydrolyzed with sodium hydroxide to give 14,
which was coupled to glycine ethyl ester hydrochloride 15 in
the presence of DCC, HOBT and triethylamine in THF providing
crude ethyl ester 16. Finally, ester 16 was hydrolyzed
with sodium hydroxide to give alvimopan (I), which was
purified by crystallization from the reaction mixture in
99.2% purity and 99% ee. 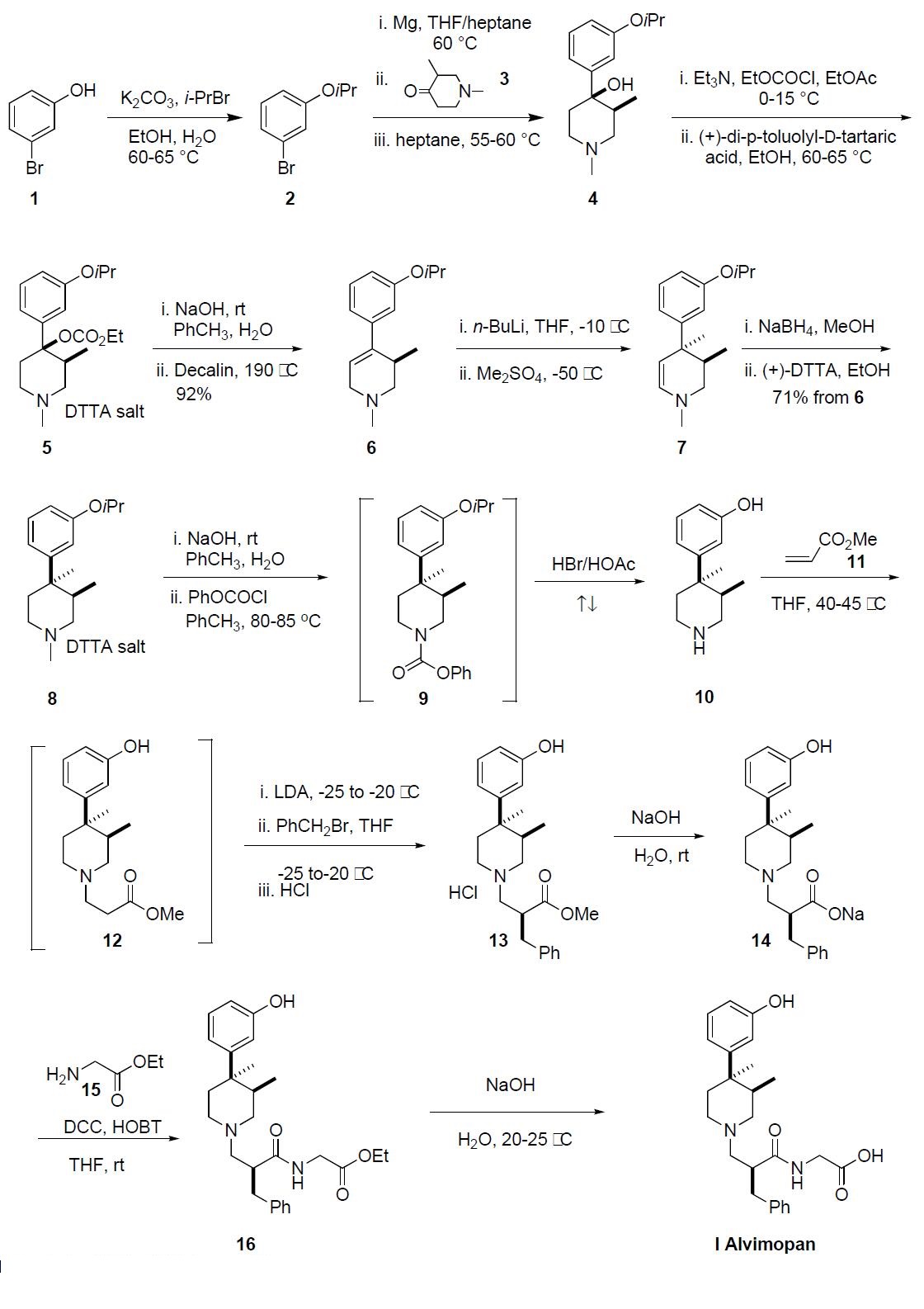
|
| | 2-[[(2S)-2-benzyl-3-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperi dyl]propanoyl]amino]acetic acid dihydrate Preparation Products And Raw materials |
|