| Company Name: |
ShangHai Caerulum Pharma Discovery Co., Ltd.
|
| Tel: |
18149758185 18149758185 |
| Email: |
sales-cpd@caerulumpharma.com |
| Products Intro: |
Product Name:Minaprine hydrochloride
CAS:25953-17-7
Purity:98% Package:1g;10g;100g
|
| Company Name: |
Musechem
|
| Tel: |
+1-800-259-7612 |
| Email: |
info@musechem.com |
| Products Intro: |
Product Name:Minaprine dihydrochloride
CAS:25953-17-7
Purity:>98% HPLC Package:10mg;100mg;1g Remarks:Reagent Grade
|
Minaprine dihydrochloride manufacturers
|
| | Minaprine dihydrochloride Basic information |
| Product Name: | Minaprine dihydrochloride | | Synonyms: | 4-Morpholineethanamine, N-(4-methyl-6-phenyl-3-pyridazinyl)-, dihydrochloride;Ag 620;Agr 1240;Agr 620;Morpholine, 4-[2-[(4-methyl-6-phenyl-3-pyridazinyl)amino]ethyl]-, dihydrochloride;Nsc305742;Pyridazine, 4-methyl-3-[(2-morpholinoethyl)amino]-6-phenyl-, dihydrochloride;4-Methyl-N-(2-morpholinoethyl)-6-phenylpyridazin-3-amine dihydrochloride | | CAS: | 25953-17-7 | | MF: | C17H24Cl2N4O | | MW: | 371.30466 | | EINECS: | | | Product Categories: | | | Mol File: | 25953-17-7.mol | 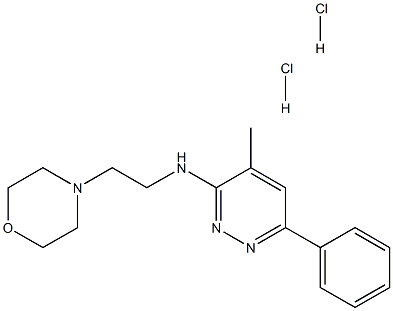 |
| | Minaprine dihydrochloride Chemical Properties |
| Melting point | 182° | | storage temp. | Store at -20°C | | solubility | DMSO : ≥ 100 mg/mL (269.32 mM) | | form | Solid | | color | White to off-white |
| Toxicity | LD50 intraperitoneal in mouse: 63mg/kg |
| | Minaprine dihydrochloride Usage And Synthesis |
| Originator | Cantor,Clin Midy,France,1979 | | Uses | Antidepressant. | | Manufacturing Process | (a) Preparation of the free base: A mixture comprising 0.1 mol (20.4 g) of 3-chloro-4-methyl-6-phenylpyridazine and 0.2 mol (26.2 g) of N-(2-aminoethyl)-morpholine in 100 ml of n-butanol, with a pinch of copper powder, was heatedunder reflux for 12 hours. At the end of this time, the hot solution was pouredinto 200 ml of cold water. The resulting mixture was filtered through asintered glass filter and the precipitate washed with ether. The filtrate and theether washings were placed in a separating funnel and extracted with two 150ml portions of ether. The ethereal layer was then extracted with about 250 mlof N sulfuric acid.
The acid solution was made alkaline with a 10% aqueous solution of sodiumcarbonate, and left to crystallize overnight.
The solution was filtered, yielding the colorless needles which wererecrystallized from isopropanol. The yield was 15 g (53%).
(b) Preparation of the hydrochloride: The base was dissolved in the smallestamount possible of anhydrous acetone. Double that volume of anhydrousether was added, and a stream of hydrogen chloride gas was passed throughthe solution. The hydrochloride salt obtained was recrystallized from absolutealcohol. The yield after recrystallization was 17 g (90%). | | Therapeutic Function | Antidepressant |
| | Minaprine dihydrochloride Preparation Products And Raw materials |
|