|
|
| | LCZ 696 Impurity C Basic information |
| Product Name: | LCZ 696 Impurity C | | Synonyms: | LCZ 696 Impurity C;(2S,4S)-Sacubitril;4-(((2S,4S)-1-([1,1'-biphenyl]-4-yl)-5-ethoxy-4-methyl-5-oxopentan-2-yl)amino)-4-oxobutanoic acid;valsartan/sacubitril Impurity 2;[1,1'-Biphenyl]-4-pentanoic acid, γ-[(3-carboxy-1-oxopropyl)amino]-α-methyl-, ethyl ester, [S-(R*,R*)]- (9CI);Sacubitril Impurity C;[1,1'-Biphenyl]-4-pentanoic acid, γ-[(3-carboxy-1-oxopropyl)amino]-α-methyl-, α-ethyl ester, (αS,γS)-;LCZ696 impuritiesC | | CAS: | 149709-63-7 | | MF: | C24H29NO5 | | MW: | 411.49 | | EINECS: | | | Product Categories: | | | Mol File: | 149709-63-7.mol | 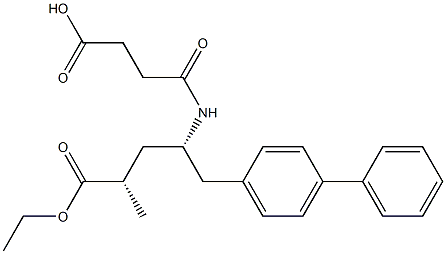 |
| | LCZ 696 Impurity C Chemical Properties |
| Boiling point | 656.9±55.0 °C(Predicted) | | density | 1.151±0.06 g/cm3(Predicted) | | storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Solid | | pka | 4.72±0.10(Predicted) | | color | White to Pale Yellow | | Stability: | Moisture Sensitive |
| | LCZ 696 Impurity C Usage And Synthesis |
| Uses | (2S,4S)-Sacubitril is an impurity of Sacubitril (S080895) which is an antihypertensive drug used in combination with valsartan. |
| | LCZ 696 Impurity C Preparation Products And Raw materials |
|