- Flupenthixol
-

- $2500.00 / 100mg
-
2024-10-28
- CAS:2709-56-0
- Min. Order:
- Purity:
- Supply Ability: 10g
|
| | Flupentiol Basic information |
| Product Name: | Flupentiol | | Synonyms: | 4-(3-(2-(trifluoromethyl)thioxanthen-9-ylidene)propyl)-1-piperazineethano;4-(3-(2-(trifluoromethyl)thioxanthen-9-ylidene)propyl)-1-piperazineethanol;emergil;fluanxol;flupenthixol;flupenthixole;fluxanxol;CIS(Z)-FLUPENTIXOL 2HCL | | CAS: | 2709-56-0 | | MF: | C23H25F3N2OS | | MW: | 434.52 | | EINECS: | 2203049 | | Product Categories: | | | Mol File: | 2709-56-0.mol | 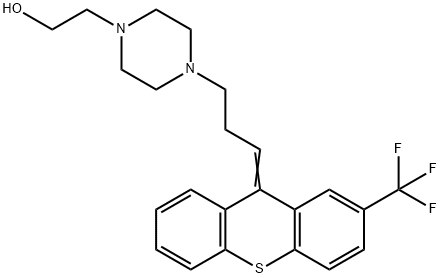 |
| | Flupentiol Chemical Properties |
| Boiling point | 554.7±50.0 °C(Predicted) | | density | 1.2118 (estimate) | | solubility | H2O: soluble | | form | solid | | pka | pKa 7.80 (Uncertain) | | color | white or off-white | | NIST Chemistry Reference | Flupentixol(2709-56-0) |
| Hazard Codes | Xn | | Risk Statements | 20/21/22 | | Safety Statements | 36/37/39 | | RIDADR | UN 2811 6.1/PG 3 | | WGK Germany | 3 | | RTECS | TL9900000 |
| | Flupentiol Usage And Synthesis |
| Originator | Emergil ,Labaz, France,1971 | | Definition | ChEBI: A thioxanthene derivative having a trifluoromethyl substituent at the 2-position and a 3-(4-(2-hydroxyethyl)piperazin-1-yl)propylidene group at the 10-position with undefined double bond stereochemistry. | | Manufacturing Process | A mixture of 200 grams of 2-benzoyloxyethanol in 2 liters of pyridine at -5°C is treated with 275 grams of p-toluenesulfonyl chloride and the resulting mixture is stirred at 0°C for 2 hours. Water is added slowly at 0° to 5°C. Extracting with chloroform, washing the extract with dilute hydrochloric acid, water and potassium bicarbonate, and evaporating the solvent leaves benzyloxyethyl p-toluenesulfonate.
A mixture of 186 grams of the above prepared p-toluenesulfonate, 106 grams of N-ethoxycarbonylpiperazine, 44 grams of potassium carbonate and 800 ml of toluene is refluxed for 21 hours, then filtered and extracted with dilute hydrochloric acid. The extract is basified with sodium hydroxide and extracted into chloroform. Evaporation of the chloroform and distillation of the residue in vacuo gives 1-benzyloxyethyl-4-ethoxy-carbonylpiperazine, BP 153° to 156°C (0.15 mm).
Hydrolysis and decarboxylation of this ester (188 grams) is accomplished by refluxing with 155 grams of potassium hydroxide, 155 ml of water and 1,550 ml of ethanol for four days. Filtering, concentrating, adding water to the residue, acidifying with hydrochloric acid, heating to 90°C, saturating with potassium carbonate, extracting into chloroform, evaporating and distilling the chloroform gives N-benzoyloxyethylpiperazine.
A mixture of 50 grams of the above prepared piperazine, 30.1 grams of sodium carbonate and 200 ml of benzene is heated to reflux and treated with 39.5 grams of 3-bromopropanol over 1.5 hours. The resulting mixture is refluxed for 2 hours, then filtered, extracted with dilute hydrochloric acid, basified, extracted with benzene, and the extracts are concentrated and distilled to give l-benzyloxyethyl-4-(3-hydroxypropyl)-piperazine, BP 188° to 190°C (0.15 mm). The free base is converted to the dihydrochloride salt by treatment of an alcoholic solution with ethereal hydrogen chloride to separate the salt.
Thionyl chloride (67 grams) is added over 15 minutes to a mixture of 39.5 grams of the above prepared dihydrochloride salt and 400 ml of chloroform. Refluxing for 4 hours, cooling and filtering yields the dihydrochloride salt of lbenzyloxyethyl-4-(3-chloropropyl)-piperazine, MP 201° to 202°C. The salt in aqueous solution is basified. Extraction with ether and evaporation of the solvent yields the free base.
Magnesium (1.3 grams) in 8 ml of refluxing tetrahydrofuran is treated with 1 ml of ethyl bromide. A solution of 22.7 grams of l-benzyloxyethyl-4-(3chloropropyl)-piperazine in 50 ml of tetrahydrofuran is added slowly and the mixture is refluxed for 1 hour.
A solution of 13.2 grams of 2-trifluoromethyl-9-xanthenone in tetrahydrofuran is added over 1 hour to 16.0 grams of 3-(4-benzyloxyethyl-1-piperazinyl) propylmagnesium chloride, prepared as above, in tetrahydrofuran while gentlyrefluxing. Refluxing is continued for 2 hours. Concentrating, pouring the residue into ammonium chloride, ice and water, extracting with ether, evaporating the extracts and treating the residue with concentrated hydrochloric acid at 95°C for 1 hour gives a mixture of cis and trans 9-[3-(4hydroxyethyl-1-piperazinyl)propylidene]-2-trifluoromethylxanthene dihydrochloride. Fractional crystallization from ethanol-ether separates the isomers. The free bases are obtained by neutralizing an aqueous solution of the dihydrochloride, extracting into ether and evaporating the ether in vacuo. | | Therapeutic Function | Tranquilizer |
| | Flupentiol Preparation Products And Raw materials |
|