| Company Name: |
ChemStrong Scientific Co.,Ltd
|
| Tel: |
0755-0755-66853366 13670046396 |
| Email: |
sales@chem-strong.com |
| Products Intro: |
Product Name:Anastrozole Impurity 15
CAS:1186102-55-5
Purity:95% HPLC Package:10mg;25mg;50mg;100mg
|
| Company Name: |
Hubei Yangxin Medical Technology Co., Ltd.
|
| Tel: |
15374522761 |
| Email: |
3003392093@qq.com |
| Products Intro: |
Product Name:Anastrozole Impurity 18
CAS:1186102-55-5
Purity:95% HPLC Package:10mg;25mg;50mg;100mg
|
| Company Name: |
Shenzhen Botel Biotechnology Co. Ltd.
|
| Tel: |
0755-22202135 13316968096 |
| Email: |
1979313431@qq.com |
| Products Intro: |
Product Name:Anastrozole Impurity 17
CAS:1186102-55-5
Purity:98% HPLC Package:10mg;25mg;50mg
|
| Company Name: |
Hefei fuya biotechnology co. LTD
|
| Tel: |
18096409024 |
| Email: |
sales02@foreversyn.com |
| Products Intro: |
Product Name:Anastrozole Impurity
CAS:1186102-55-5
Package:100mg;250mg
|
|
| | Anastrozole Impurity 18 Basic information |
| Product Name: | Anastrozole Impurity 18 | | Synonyms: | Anastrozole Impurity 18;Anastrozole Impurity 13;Anastrozole Impurity 17;Anastrozole Impurity 22;2,2''-(5-(di(1H-1,2,4-Triazol-1-yl)methyl)-1,3-phenylene)bis(2-methylpropanenitrile);1,3-Benzenediacetonitrile, 5-[bis(1H-1,2,4-triazol-1-yl)methyl]-α1,α1,α3,α3-tetramethyl- | | CAS: | 1186102-55-5 | | MF: | C19H20N8 | | MW: | 360.42 | | EINECS: | | | Product Categories: | | | Mol File: | 1186102-55-5.mol | 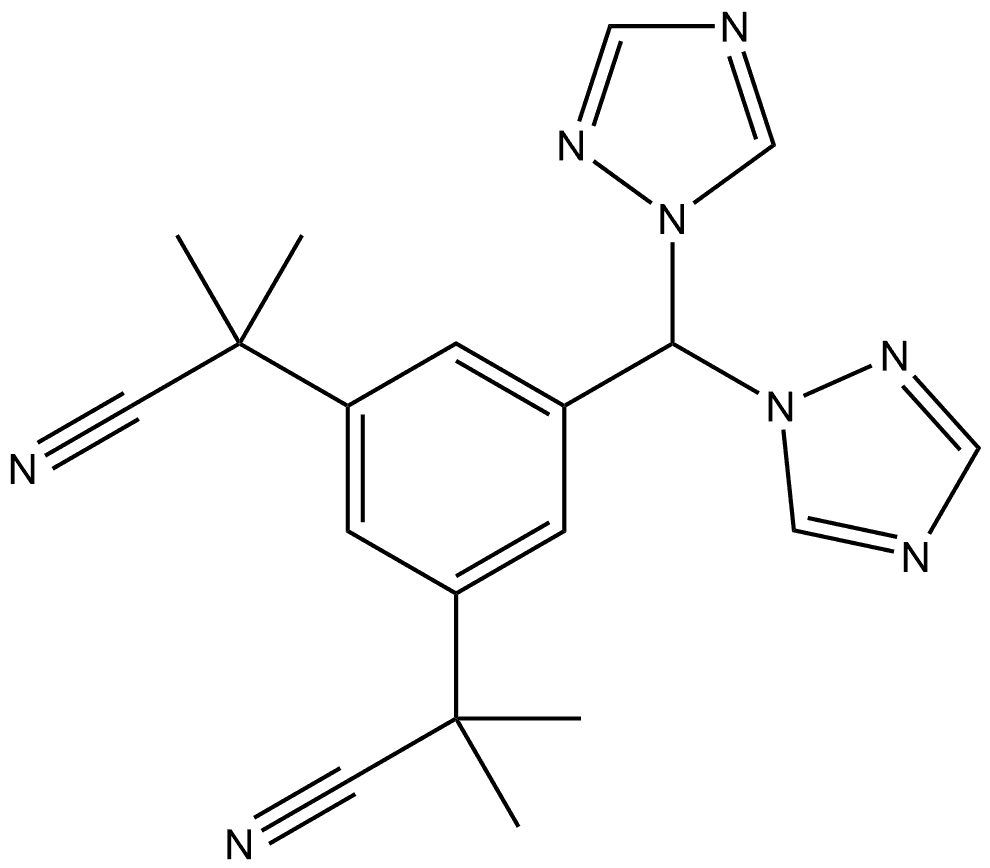 |
| | Anastrozole Impurity 18 Chemical Properties |
| Boiling point | 575.5±60.0 °C(Predicted) | | density | 1.22±0.1 g/cm3(Predicted) | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Solid | | pka | 1.39±0.11(Predicted) | | color | White to Off-White |
| | Anastrozole Impurity 18 Usage And Synthesis |
| Uses | 5-[Bis(1H-1,2,4-triazol-1-yl)methyl]-α1,α1,α3,α3-tetramethyl-1,3-benzenediacetonitrile is used in LC and LC-?MS?/MS study of forced decomposition behavior of anastrozole and establishment of validated stability-?indicating analytical method for impurities estimation in low dose anastrozole tablets. |
| | Anastrozole Impurity 18 Preparation Products And Raw materials |
|