|
|
| | (±)-2-[4-[3-[[4-benzamido-5-(dipropylamino)glutaryl]oxy]propyl]-1-piperazinyl]ethyl 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetate, compound with maleic acid (1:2) Basic information |
| Product Name: | (±)-2-[4-[3-[[4-benzamido-5-(dipropylamino)glutaryl]oxy]propyl]-1-piperazinyl]ethyl 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetate, compound with maleic acid (1:2) | | Synonyms: | 1H-Indole-3-acetic acid, 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-, 2-[4-[3-[[4-(benzoylamino)-5-(dipropylamino)-1,5-dioxopentyl]oxy]propyl]-1-piperazinyl]ethyl ester, (+-)-, (Z)-2-butenedioate (1:2);1H-Indole-3-acetic acid, 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-, 2-[4-[3-[[4-(benzoylamino)-5-(dipropylamino)-1,5-dioxopentyl]oxy]propyl]-1-piperazinyl]ethyl ester, (2Z)-2-butenedioate (1:2) (9CI);1H-Indole-3-acetic acid, 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-, 2-[4-[3-[[4-(benzoylamino)-5-(dipropylamino)-1,5-dioxopentyl]oxy]propyl]-1-piperazinyl]ethyl ester, (Z)-2-butenedioate (1:2);Afloxan;CR 604;Miridacin;Protacine;Protaxon | | CAS: | 59209-40-4 | | MF: | C46H58ClN5O8.2C4H4O4 | | MW: | 0 | | EINECS: | 261-656-3 | | Product Categories: | | | Mol File: | Mol File | ![(±)-2-[4-[3-[[4-benzamido-5-(dipropylamino)glutaryl]oxy]propyl]-1-piperazinyl]ethyl 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetate, compound with maleic acid (1:2) Structure]() |
| | (±)-2-[4-[3-[[4-benzamido-5-(dipropylamino)glutaryl]oxy]propyl]-1-piperazinyl]ethyl 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetate, compound with maleic acid (1:2) Chemical Properties |
| Toxicity | LD50 in male mice, rats (mg/kg): 262, 450 orally (Rovati) |
| | (±)-2-[4-[3-[[4-benzamido-5-(dipropylamino)glutaryl]oxy]propyl]-1-piperazinyl]ethyl 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetate, compound with maleic acid (1:2) Usage And Synthesis |
| Definition | ChEBI: A maleate salt obtained by combining proglumetacin with two molar equivalents of maleic acid. Used to control pain and inflammation associated with musculoskeletal and joint disorders. Following oral adminitration, it is metabolied to indometacin and progl
mide, a drug with antisecretory effects that helps prevent injury to the stomach lining. | | General Description | Proglumetacin at a dose of 450 mg/d appears
to be as effective as 150 mg/d of indomethacin
in the treatment of a wide variety of rheumatic
conditions, and is faster acting with significantly
lower incidence and severity of adverse effects.
It is metabolized to several derivatives, including
indomethacin, in humans. | | Trade name | Afloxan (Rotta Research,
Italy), Protaxil (Rottapharm, Spain), Protaxon
(Opfermann, Germany), Proxil (Rottapharm,
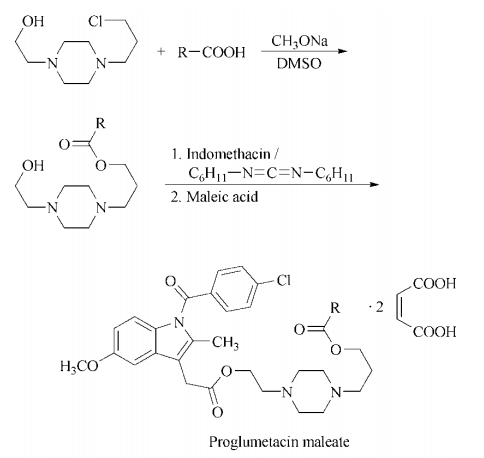
Italy), Tolindol (La Meuse, Belgium). | | Synthesis | alkylation of the anticholinergic agent proglumide with N-(3-chloropropyl)-N'-(2-hydroxyethyl)piperazine using
sodium methoxide in dimethyl sulfoxide gives N-[3-(N-benzoyl-N' ,N '-di-npropyl-dl-isoglutaminyl)oxypropyl]-N'-(2-
hydroxyethyl)piperazine, which is then condensed with indomethacin using dicyclohexylcarbodiimide. The resulting ester is treated
with maleic acid to give proglumetacin maleate.
 |
| | (±)-2-[4-[3-[[4-benzamido-5-(dipropylamino)glutaryl]oxy]propyl]-1-piperazinyl]ethyl 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetate, compound with maleic acid (1:2) Preparation Products And Raw materials |
|