| Company Name: |
Shaanxi Dideu Newmaterial Co., Ltd.
|
| Tel: |
029-87576359 15353716720 |
| Email: |
1039@dideu.com |
| Products Intro: |
Product Name:Radium dichloride
CAS:10025-66-8
Purity:98% Package:25kg
|
|
| | radium chloride Basic information |
| Product Name: | radium chloride | | Synonyms: | radium chloride;Radium dichloride;Einecs 233-035-7;Radium chloride (RaCl2)(9CI) | | CAS: | 10025-66-8 | | MF: | Cl2Ra | | MW: | 296.906 | | EINECS: | 2330357 | | Product Categories: | | | Mol File: | 10025-66-8.mol | 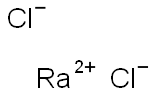 |
| | radium chloride Chemical Properties |
| Melting point | 1000° | | density | 4.91 | | solubility | soluble in ethanol | | form | white orthorhombic crystals | | color | white orthorhombic crystals, crystalline | | Water Solubility | soluble H2O [MER06]; soluble alcohol [HAW93] |
| | radium chloride Usage And Synthesis |
| Description | Radium chloride, RaCl2, was the first radium
compound to be prepared in a pure state and was the
basis of Marie Curie’s original separation of radium
from Barium. The first preparation of radium metal
was by the electrolysis of a solution of radium chloride
using a mercury cathode. The structure remains
unknown.
Radium chloride is soluble and crystallizes from solution
as the dihydrate.
RaCl2 is used to produce radon gas that in turn is
used as a cancer treatment in medicine. This compound
is not offered for sale to the general public. | | Chemical Properties | radium chloride is yellowish-white crystals becoming yel- low or pink on standing. Radiactive. Soluble in water and alcohol. | | Physical properties | radium chloride is a white solid having a blue-green luminescence, especially when heated. It is less soluble in water than other alkaline earth chlorides, a fact which is used in the first stages of the separation of radium from barium by fractional crystallization. It is only sparingly soluble in azeotropic HCl (20.2% HCl) and virtually insoluble in concentrated hydrochloric acid. | | Uses | Radium chloride (RaCl2) has the same use as radium bromide. | | Uses | In physical research. In radiography of metals because the penetration of gamma rays is more pronounced than that of x-rays. As source of radon. No longer used to make luminous paints. | | Hazard | As for radium. |
| | radium chloride Preparation Products And Raw materials |
|