Ethyl (R)-(+)-2-(4-hydroxyphenoxy)propionate manufacturers
|
| | Ethyl (R)-(+)-2-(4-hydroxyphenoxy)propionate Basic information |
| Product Name: | Ethyl (R)-(+)-2-(4-hydroxyphenoxy)propionate | | Synonyms: | Ethyl(R)-(+)-2-(4-hydroxyphenoxy)propanoate;(R)-ethyl 2-(4-hydroxyphenoxy)propanoate;(R)-2-(4-Hydroxyphenoxy)propionic acid ethyl ester;(R)-2-(p-Hydroxyphenoxy)propionic acid ethyl ester;Propanoic acid, 2-(4-hydroxyphenoxy)-, ethyl ester, (2R)-;Ethyl 2(R)-(4-Hydroxy-phenoxy)-propionate | | CAS: | 71301-98-9 | | MF: | C11H14O4 | | MW: | 210.23 | | EINECS: | | | Product Categories: | | | Mol File: | 71301-98-9.mol | 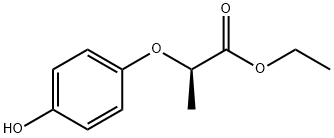 |
| | Ethyl (R)-(+)-2-(4-hydroxyphenoxy)propionate Chemical Properties |
| Melting point | 36-37℃ | | Boiling point | 327℃ | | density | 1.156 | | Fp | 124℃ | | storage temp. | 2-8°C | | pka | 10.09±0.15(Predicted) |
| | Ethyl (R)-(+)-2-(4-hydroxyphenoxy)propionate Usage And Synthesis |
| Uses | Ethyl (R)-(+)-2-(4-hydroxyphenoxy)propionate is an organic intermediate compound used mainly as a raw material for the synthesis of other compounds or for chemical research. It is also a degradation product of the herbicide fenoxaprop-ethyl (FE) in water, sediment and water-sediment microenvironments. | | Synthesis | Ethyl (R)-(+)-2-(4-hydroxyphenoxy)propionate is prepared by the reaction of hydroquinone and (R)-ethyl O-bezenesulfonyl lactate in the presence of inert atmosphere. The steps are as follows:
Add hydroquinone (8.25g, 0.075mol), sodium carbonate (6.36g, 0.06mol), PEG-1500 (0.5g) into a 250mL nitrogen-proof flask equipped with a magnetic stir bar, and pass the nitrogen-proof flask into The nitrogen is then pumped with an oil pump and then fed into nitrogen (three consecutive times), and then 80 mL of xylene is added, and the reaction flask is placed in an oil bath heated to 120°C for 1 hour.Then add dropwise the xylene dilution liquid of ethyl lactate benzenesulfonate (12.9g, 0.05mol/20ml xylene, add three times, add once in 10 minutes, and add the next in 30 minutes), and then react after the addition is complete. The 6h reaction is over; the reaction solution is suction filtered, an appropriate amount of water is added to the suction filtrate, the solution is extracted with dichloromethane (washed and separated three times), dried with anhydrous sodium sulfate, and finally the solvent is removed by a rotary evaporator to obtain a light yellow Transparent crystals (containing a small amount of hydroquinone), 9.1 g of Ethyl (R)-(+)-2-(4-hydroxyphenoxy)propionate was obtained by column chromatography, and the yield was 86.7%. Where Ar represents that the reaction system is always isolated from oxygen, that is, inert gas protection is used.
 |
| | Ethyl (R)-(+)-2-(4-hydroxyphenoxy)propionate Preparation Products And Raw materials |
|