|
|
| Product Name: | ClO2F | | Synonyms: | ClO2F;CHLORYL FLUORIDE);Chloryl fluoride ((ClO2)F) (9CI) | | CAS: | 13637-83-7 | | MF: | ClFO2 | | MW: | 86.45 | | EINECS: | | | Product Categories: | | | Mol File: | 13637-83-7.mol | 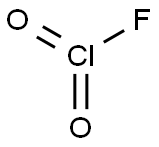 |
| | ClO2F Chemical Properties |
| Melting point | -115℃ [CRC10] | | Boiling point | -5.64°C (estimate) | | solubility | reacts with H2O | | form | gas | | color | colorless | | Water Solubility | reac H2O [CRC10]Density, g/L: 3.534 [CRC10] |
| RIDADR | 1955 | | HazardClass | 2.3 |
| | ClO2F Usage And Synthesis |
| Chemical Properties | col gas [CRC10] | | Production Methods |
Fluorine flows at a rate of 500 ml/hr into the first trap, where a few milliliters of liquid ClO2 at —50 to —55°C have been placed. The inlet tube dips a few millimeters into the liquid ClO2. The reaction progresses smoothly and steadily; most of the ClO2F formed in the reactor remains there. When the colour of the liquid in the first trap becomes very faint, the reactor is allowed to warm, and the ClO2F is distilled into the second trap in a stream of fluorine, with a gradually rising temperature. It collects as a pure, colourless substance requiring no further purification.
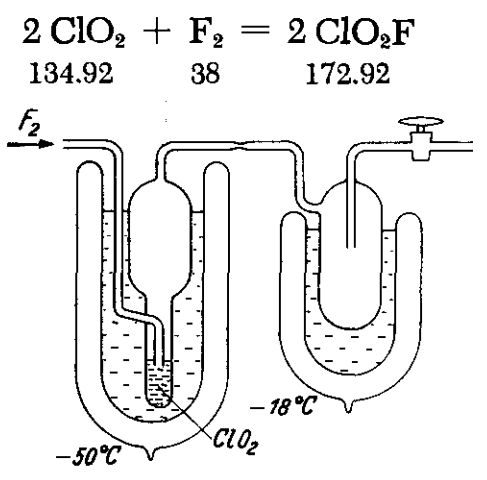
|
| | ClO2F Preparation Products And Raw materials |
|